Magnesium in PDB 7mqq: Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc)
Protein crystallography data
The structure of Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc), PDB code: 7mqq
was solved by
M.A.Outram,
D.J.Ericsson,
S.J.Williams,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 31.62 / 1.15 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.732, 50.732, 91.091, 90, 90, 120 |
| R / Rfree (%) | 16.2 / 17.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc)
(pdb code 7mqq). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc), PDB code: 7mqq:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc), PDB code: 7mqq:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 7mqq
Go back to
Magnesium binding site 1 out
of 2 in the Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc)
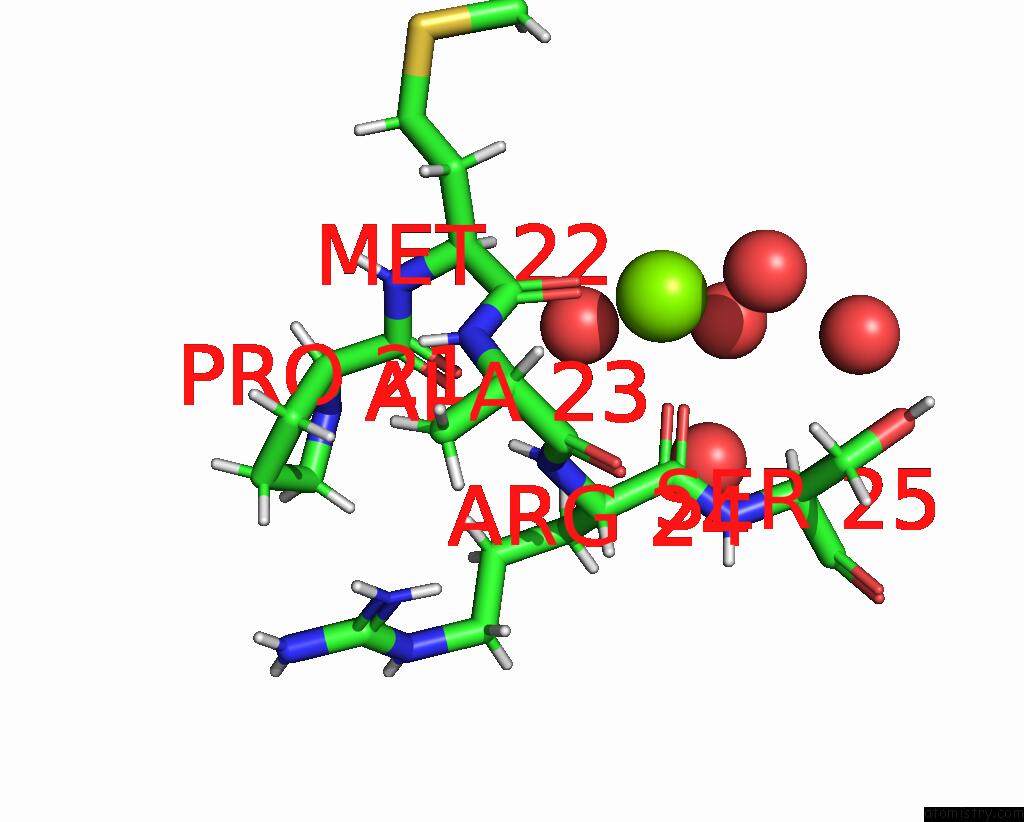
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc) within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 7mqq
Go back to
Magnesium binding site 2 out
of 2 in the Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc)

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of An Allelic Variant of Puccinia Graminis F. Sp. Tritici (Pgt) Effector AVRSR50 (Qcmjc) within 5.0Å range:
|
Reference:
D.Ortiz,
J.Chen,
M.A.Outram,
I.M.L.Saur,
N.M.Upadhyaya,
R.Mago,
D.J.Ericsson,
S.Cesari,
C.Chen,
S.J.Williams,
P.N.Dodds.
The Stem Rust Effector Protein AVRSR50 Escapes SR50 Recognition By A Substitution in A Single Surface-Exposed Residue. New Phytol. V. 234 592 2022.
ISSN: ESSN 1469-8137
PubMed: 35107838
DOI: 10.1111/NPH.18011
Page generated: Thu Oct 3 01:05:35 2024
ISSN: ESSN 1469-8137
PubMed: 35107838
DOI: 10.1111/NPH.18011
Last articles
K in 9G9VK in 9DTR
K in 9C46
K in 9G9W
K in 9G9X
K in 9ESI
K in 9ESH
K in 8ZEX
K in 8VAV
K in 8VAZ