Magnesium in PDB 8qmp: Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8
Enzymatic activity of Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8
All present enzymatic activity of Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8:
7.2.2.10;
7.2.2.10;
Other elements in 8qmp:
The structure of Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8 also contains other interesting chemical elements:
| Fluorine | (F) | 3 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8
(pdb code 8qmp). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8, PDB code: 8qmp:
In total only one binding site of Magnesium was determined in the Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8, PDB code: 8qmp:
Magnesium binding site 1 out of 1 in 8qmp
Go back to
Magnesium binding site 1 out
of 1 in the Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8
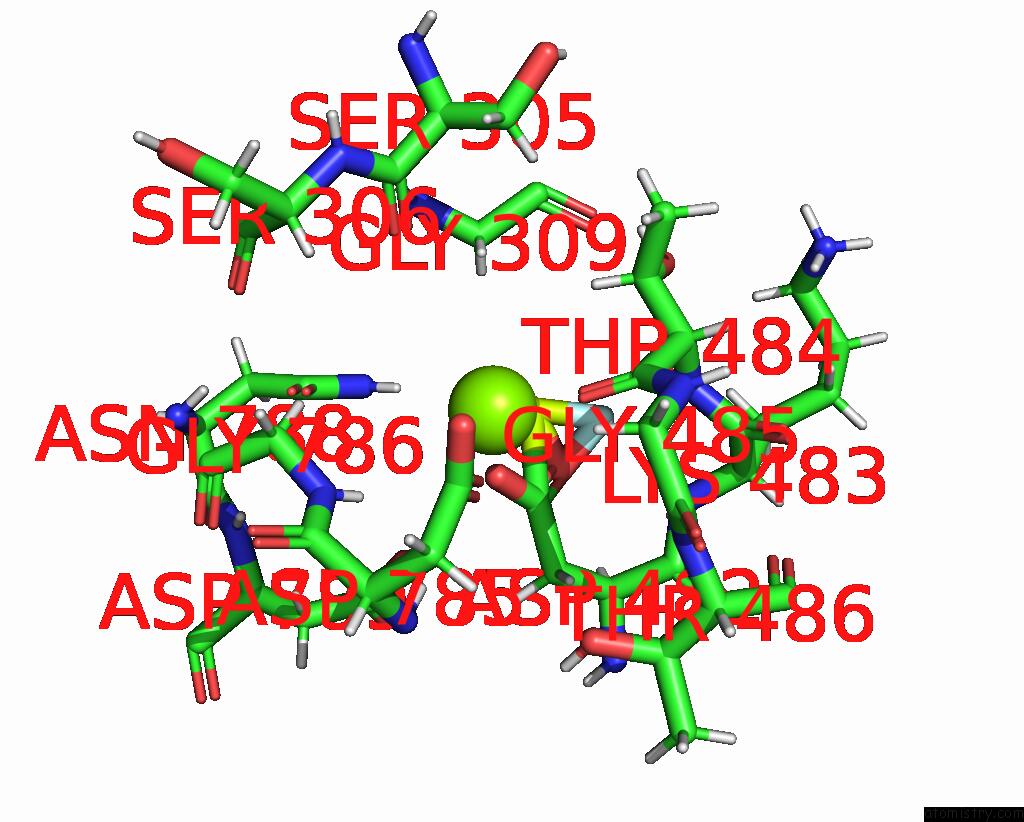
Mono view
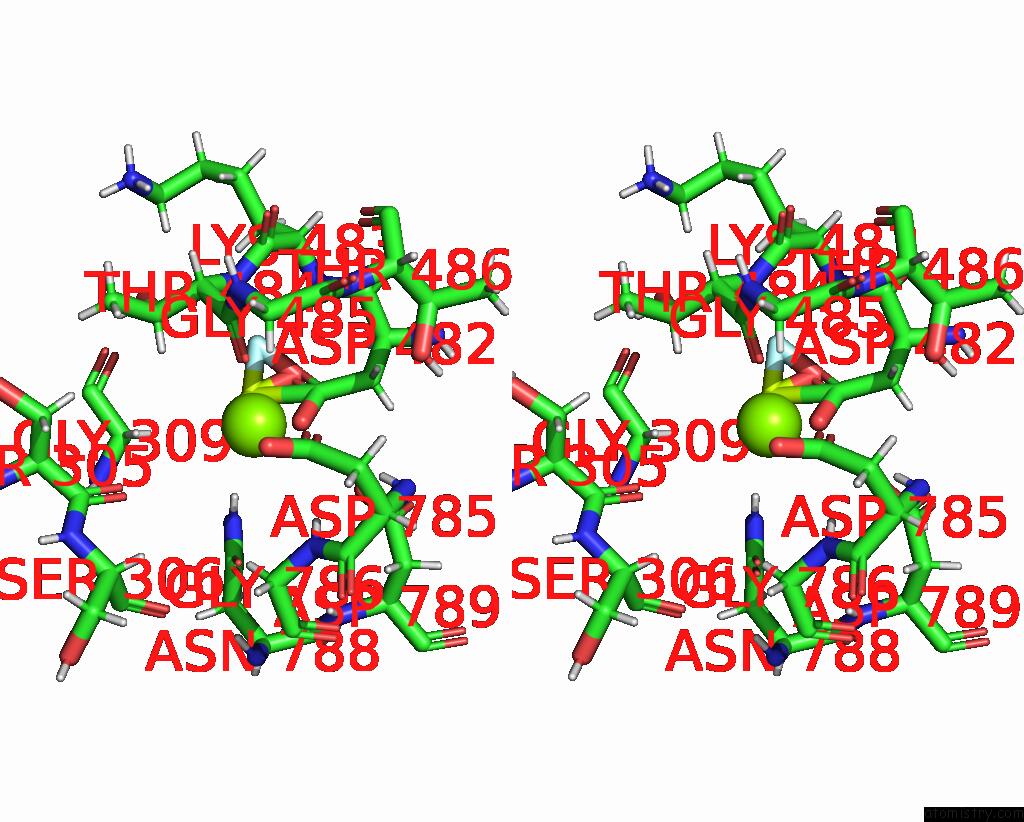
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the E2 Beryllium Fluoride Complex of the Autoinhibited Calcium Atpase ACA8 within 5.0Å range:
|
Reference:
S.T.Larsen,
J.K.Dannerso,
C.J.F.Nielsen,
L.R.Poulsen,
M.Palmgren,
P.Nissen.
Conserved N-Terminal Regulation of the ACA8 Calcium Pump with Two Calmodulin Binding Sites. J.Mol.Biol. V. 436 68747 2024.
ISSN: ESSN 1089-8638
PubMed: 39168442
DOI: 10.1016/J.JMB.2024.168747
Page generated: Thu Oct 31 21:59:10 2024
ISSN: ESSN 1089-8638
PubMed: 39168442
DOI: 10.1016/J.JMB.2024.168747
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF