Magnesium in PDB 8urp: Membrane Protein Enzyme in State 2
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Membrane Protein Enzyme in State 2
(pdb code 8urp). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Membrane Protein Enzyme in State 2, PDB code: 8urp:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Membrane Protein Enzyme in State 2, PDB code: 8urp:
Jump to Magnesium binding site number: 1; 2; 3; 4;
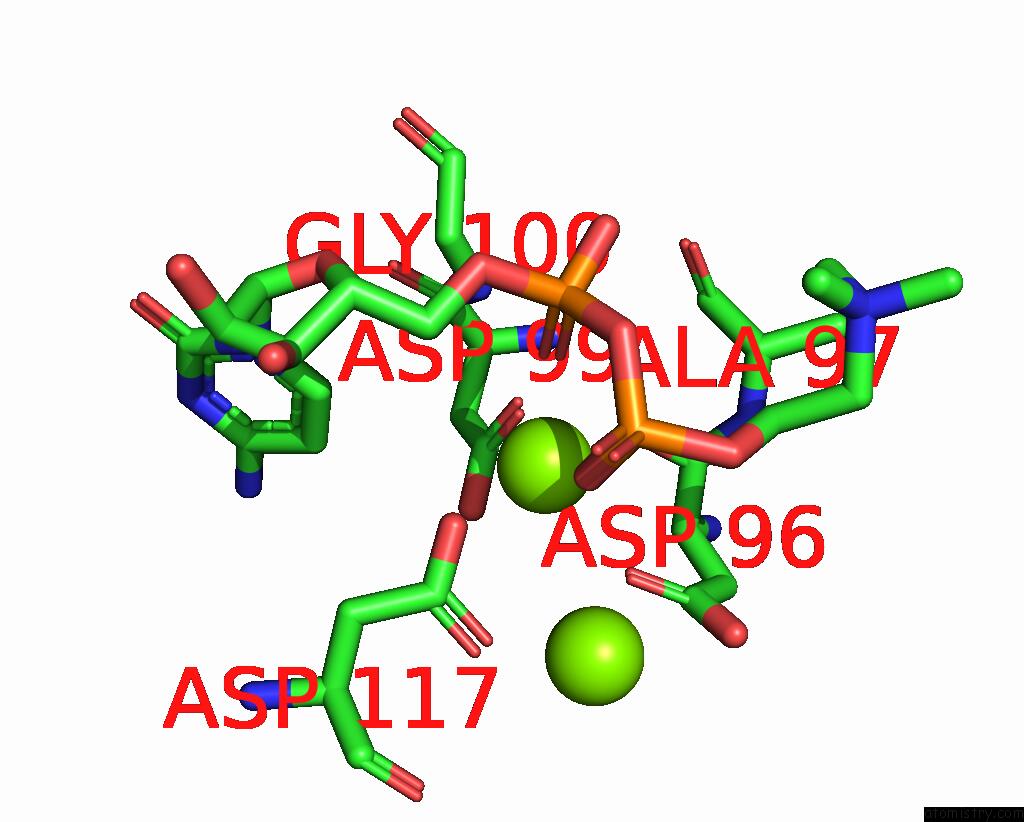
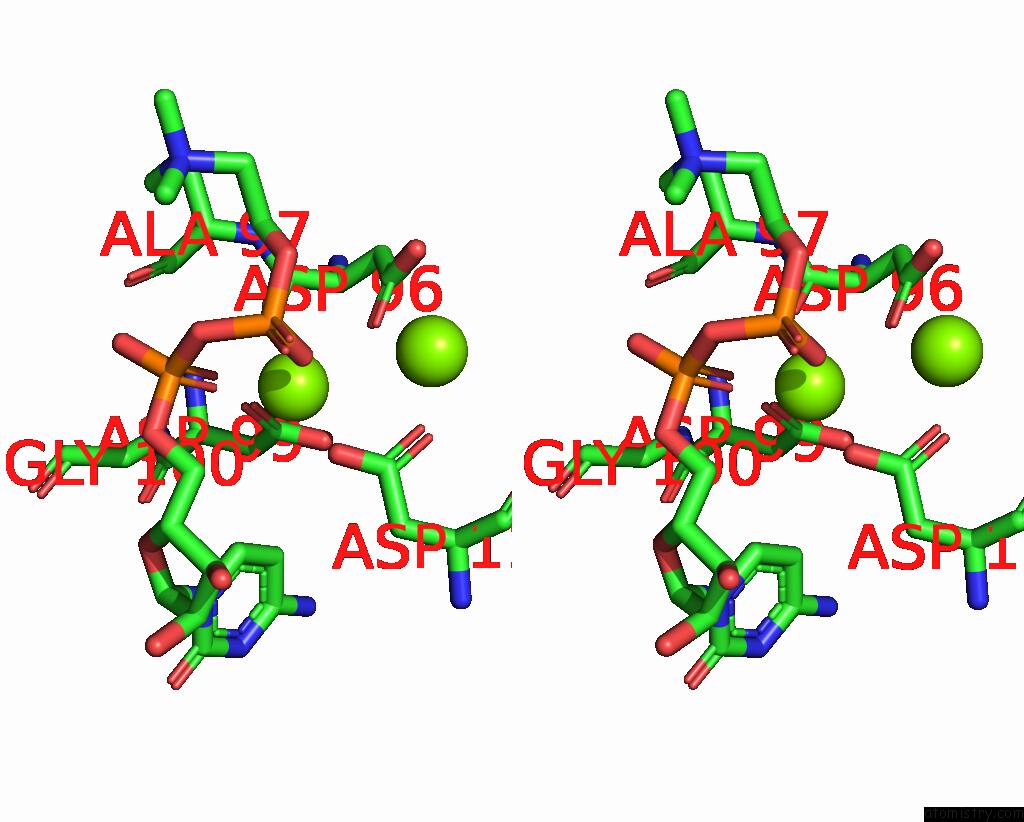
Magnesium binding site 1 out of 4 in 8urp
Go back to
Magnesium binding site 1 out
of 4 in the Membrane Protein Enzyme in State 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Membrane Protein Enzyme in State 2 within 5.0Å range:
|
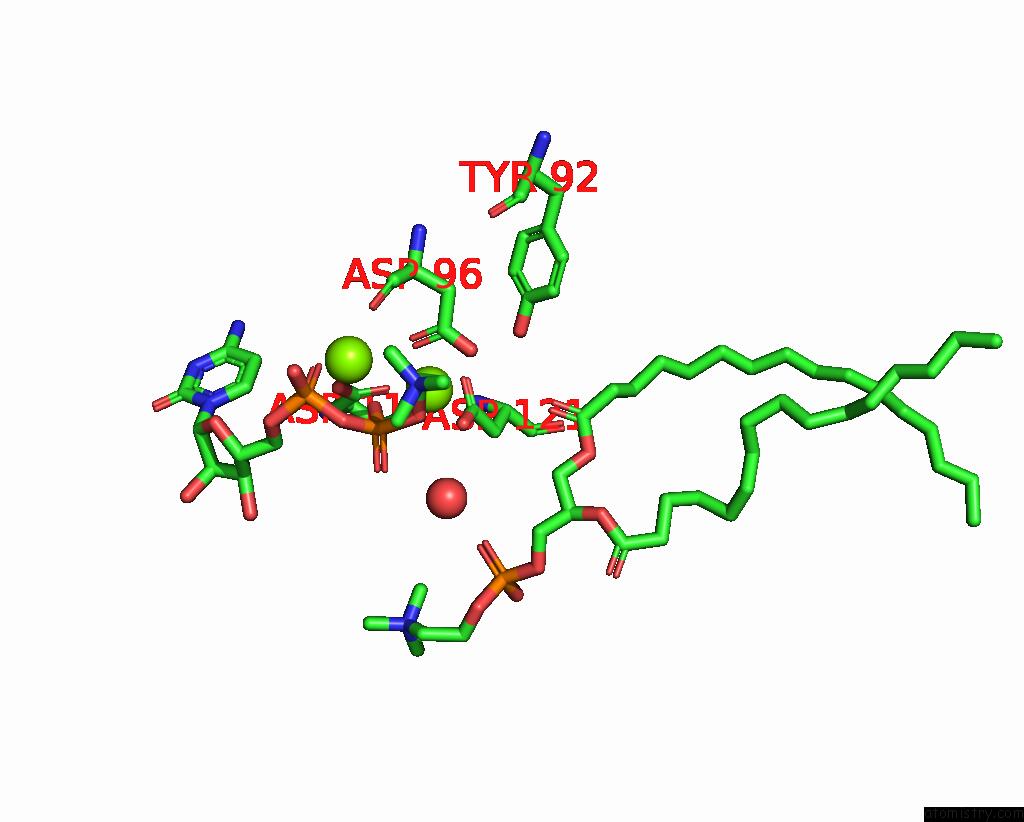
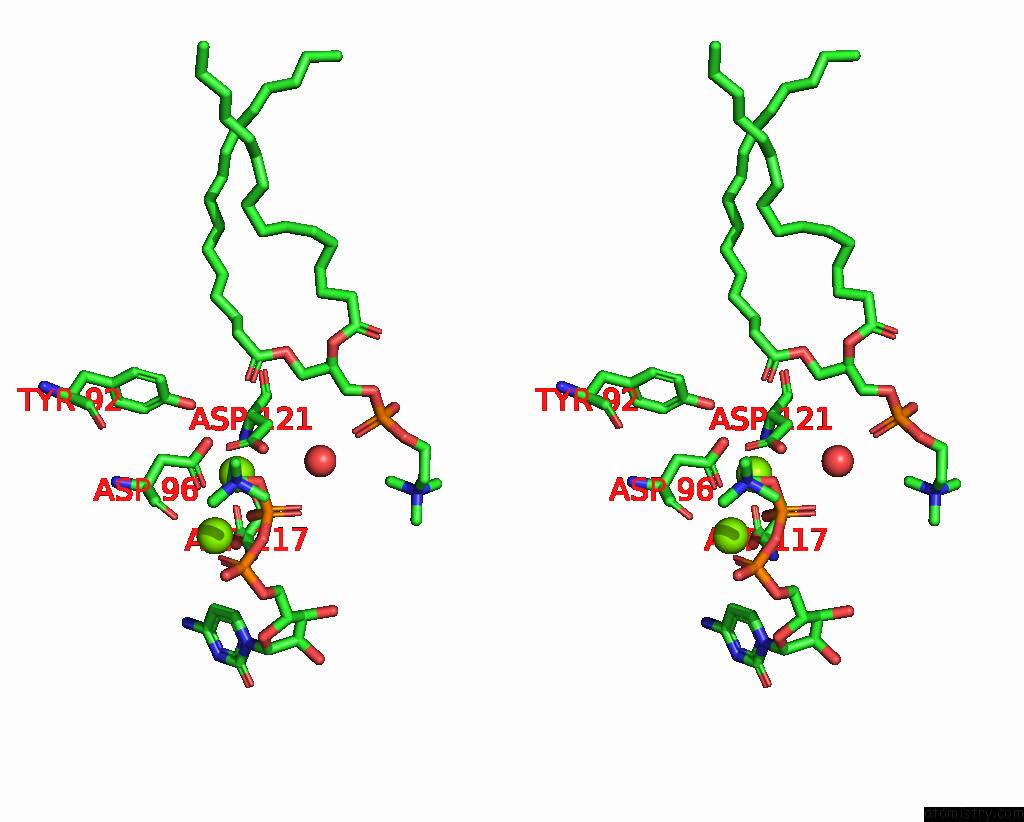
Magnesium binding site 2 out of 4 in 8urp
Go back to
Magnesium binding site 2 out
of 4 in the Membrane Protein Enzyme in State 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Membrane Protein Enzyme in State 2 within 5.0Å range:
|
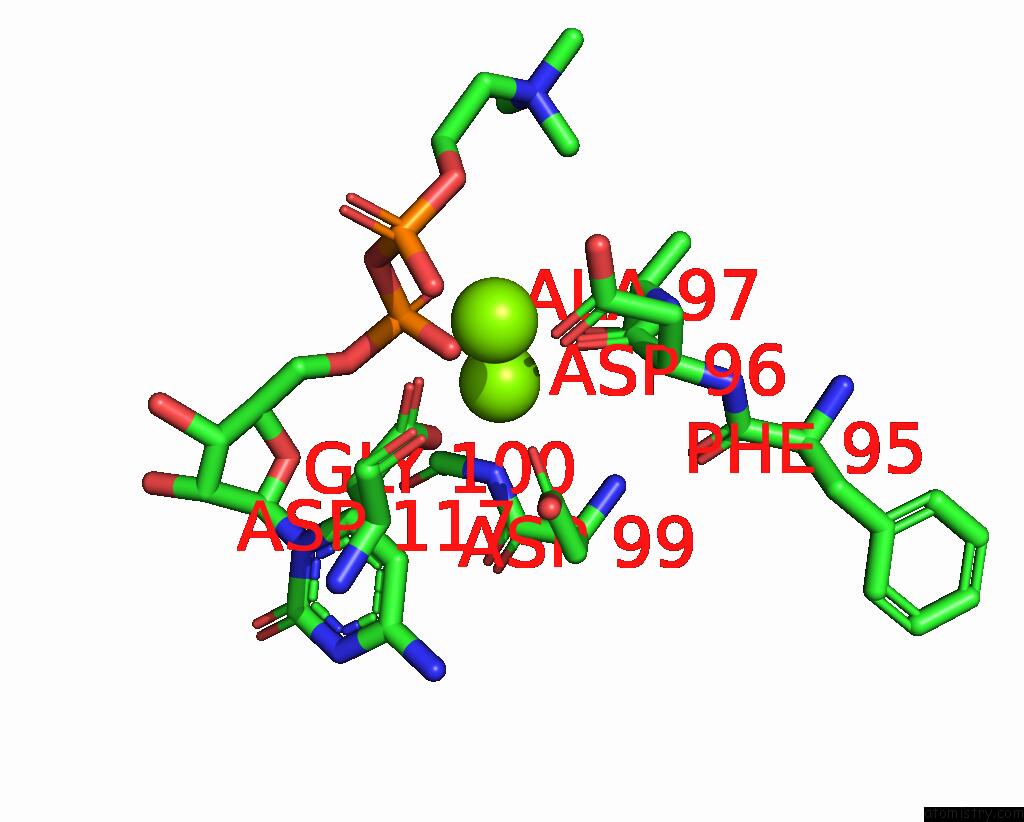
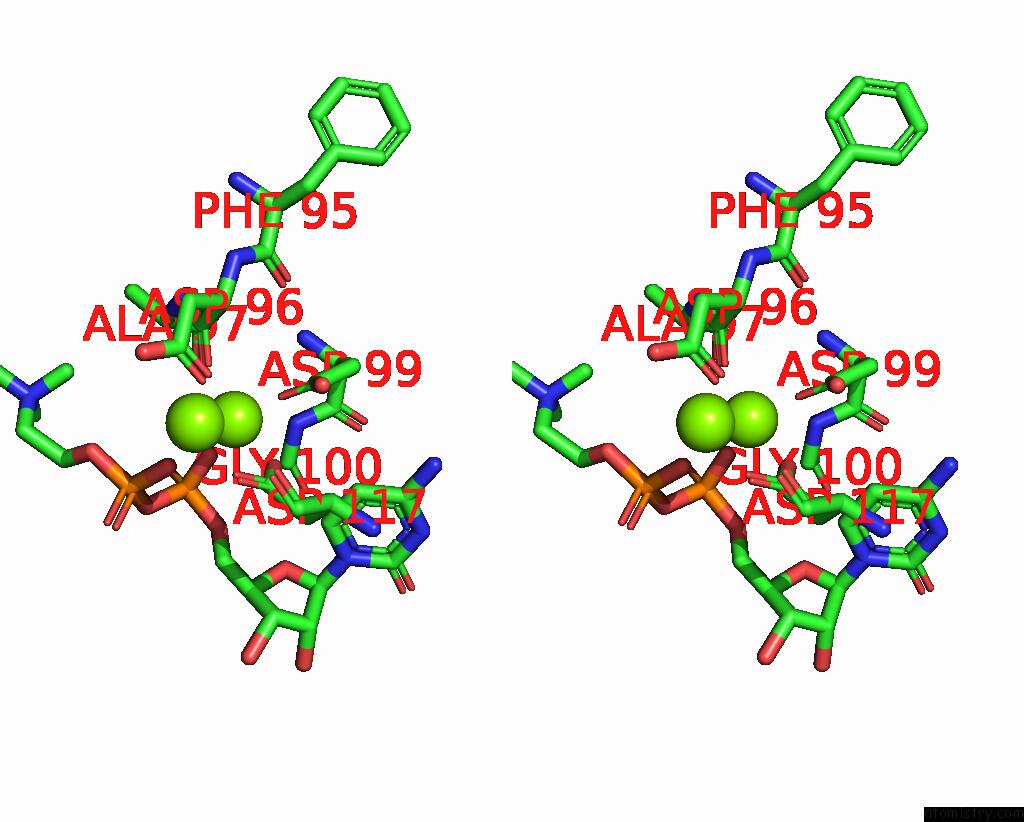
Magnesium binding site 3 out of 4 in 8urp
Go back to
Magnesium binding site 3 out
of 4 in the Membrane Protein Enzyme in State 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Membrane Protein Enzyme in State 2 within 5.0Å range:
|
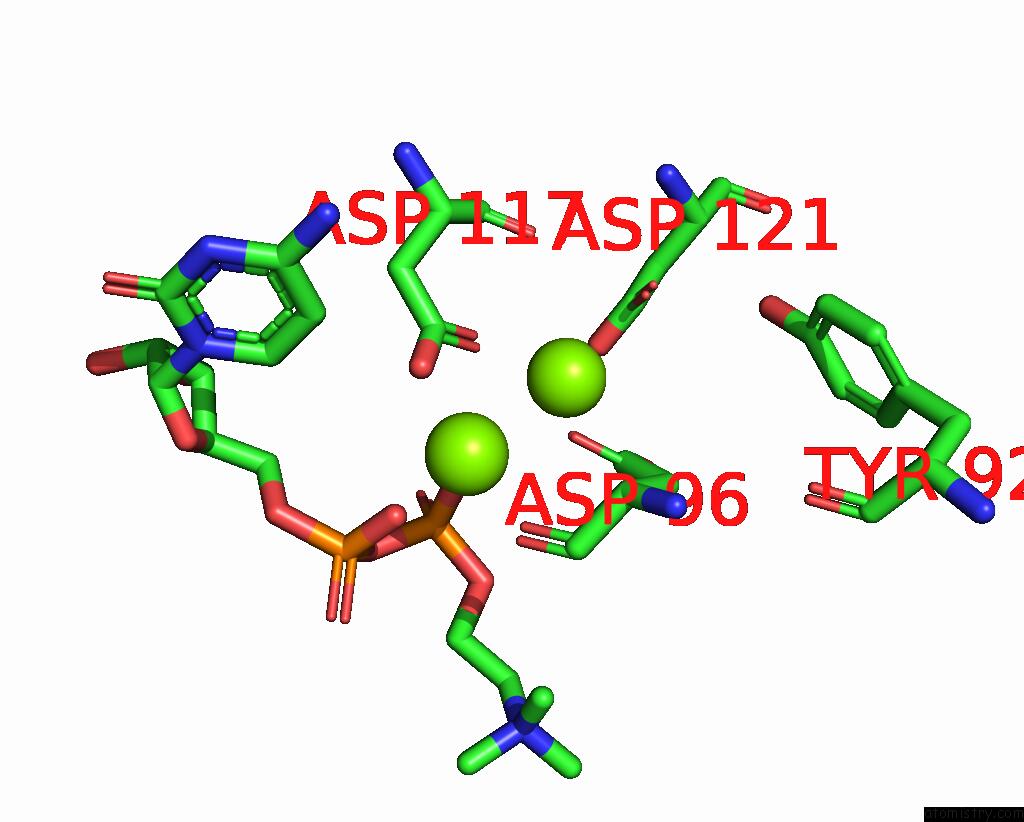
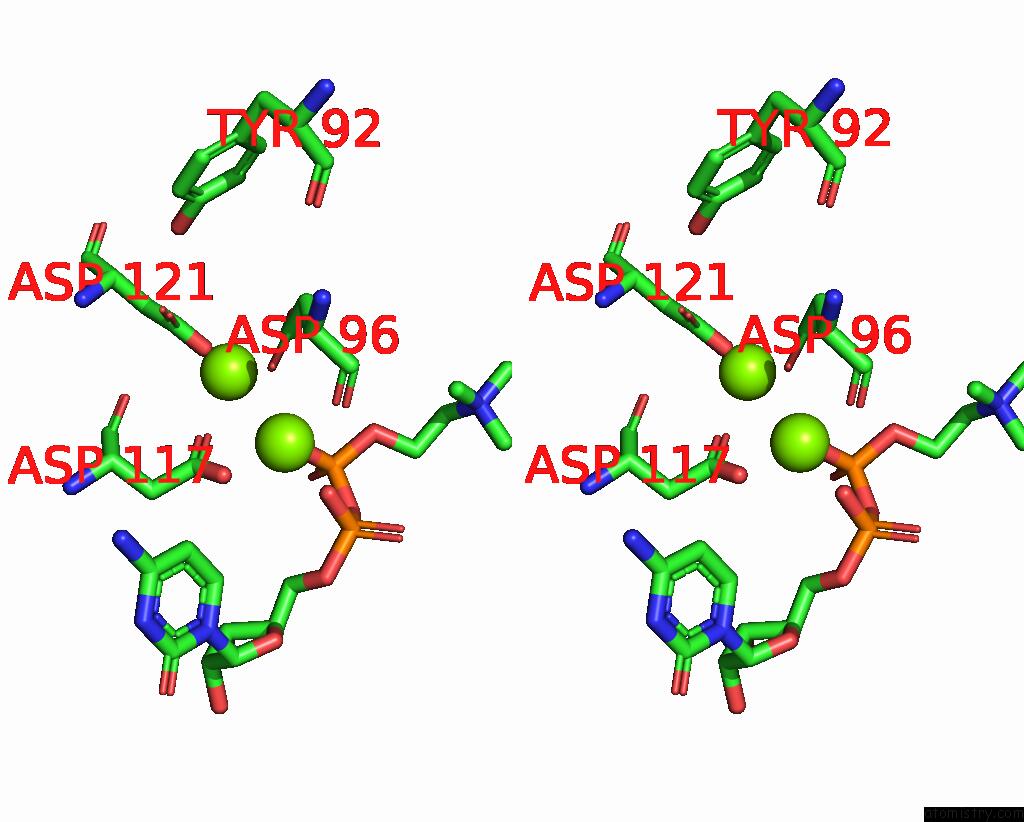
Magnesium binding site 4 out of 4 in 8urp
Go back to
Magnesium binding site 4 out
of 4 in the Membrane Protein Enzyme in State 2
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Membrane Protein Enzyme in State 2 within 5.0Å range:
|
Reference:
J.R.Roberts,
Y.Horibata,
F.Kwarcinski,
V.Lam,
A.M.Raczkowski,
A.Hubbard,
H.Sugimoto,
G.G.Tall,
M.D.Ohi,
S.Maeda.
Structural Basis For Catalysis and Selectivity of Phospholipid Synthesis By Eukaryotic Choline-Phosphotransferase To Be Published.
Page generated: Wed Nov 13 12:04:16 2024
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF