Magnesium »
PDB 1c5u-1cxz »
1cg1 »
Magnesium in PDB 1cg1: Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+
Enzymatic activity of Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+
All present enzymatic activity of Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+:
6.3.4.4;
6.3.4.4;
Protein crystallography data
The structure of Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+, PDB code: 1cg1
was solved by
J.Y.Choe,
B.W.Poland,
H.Fromm,
R.Honzatko,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 5.00 / 2.50 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 80.370, 80.370, 158.520, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 16.9 / 24.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+
(pdb code 1cg1). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+, PDB code: 1cg1:
In total only one binding site of Magnesium was determined in the Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+, PDB code: 1cg1:
Magnesium binding site 1 out of 1 in 1cg1
Go back to
Magnesium binding site 1 out
of 1 in the Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+
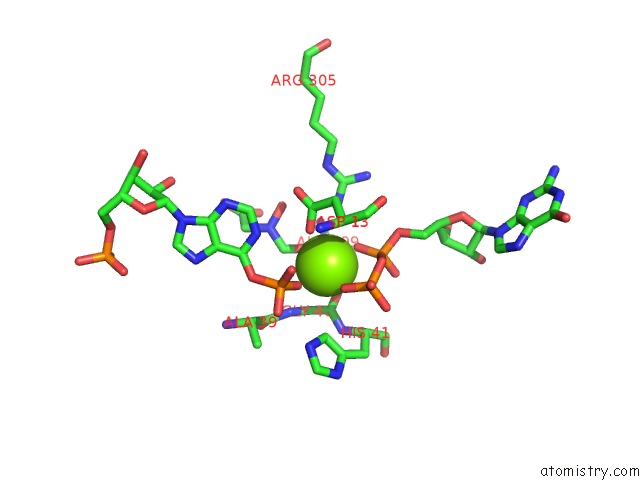
Mono view
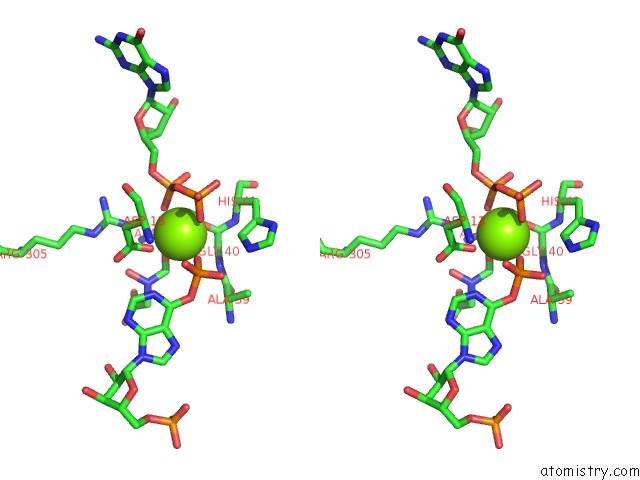
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the Mutant (K16Q) of Adenylosuccinate Synthetase From E. Coli Complexed with Hadacidin, Gdp, 6-Phosphoryl-Imp, and MG2+ within 5.0Å range:
|
Reference:
J.Y.Choe,
B.W.Poland,
H.J.Fromm,
R.B.Honzatko.
Mechanistic Implications From Crystalline Complexes of Wild-Type and Mutant Adenylosuccinate Synthetases From Escherichia Coli. Biochemistry V. 38 6953 1999.
ISSN: ISSN 0006-2960
PubMed: 10346917
DOI: 10.1021/BI990159S
Page generated: Tue Aug 13 02:28:30 2024
ISSN: ISSN 0006-2960
PubMed: 10346917
DOI: 10.1021/BI990159S
Last articles
Cl in 6BSGCl in 6BSH
Cl in 6BSI
Cl in 6BSD
Cl in 6BRD
Cl in 6BRE
Cl in 6BRY
Cl in 6BRF
Cl in 6BRA
Cl in 6BRC