Magnesium »
PDB 1eo3-1f6t »
1eyj »
Magnesium in PDB 1eyj: Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State)
Enzymatic activity of Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State)
All present enzymatic activity of Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State):
3.1.3.11;
3.1.3.11;
Protein crystallography data
The structure of Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State), PDB code: 1eyj
was solved by
J.Choe,
R.B.Honzatko,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 5.00 / 2.28 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.860, 165.820, 79.510, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.7 / 25.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State)
(pdb code 1eyj). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State), PDB code: 1eyj:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State), PDB code: 1eyj:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1eyj
Go back to
Magnesium binding site 1 out
of 2 in the Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State)
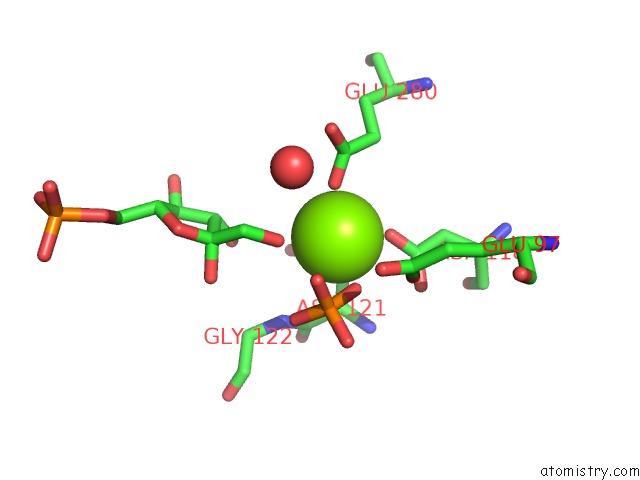
Mono view
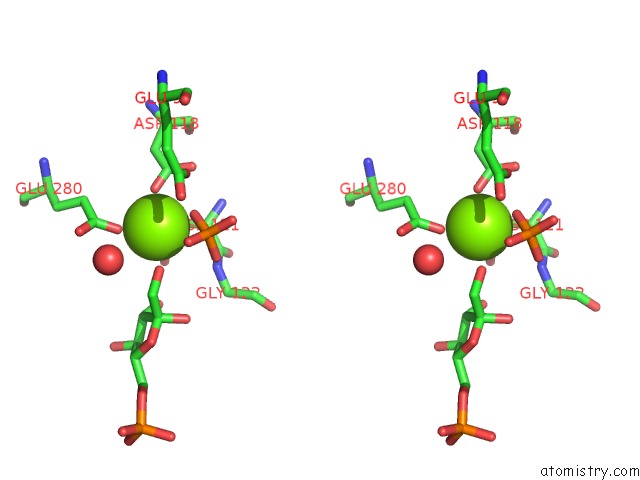
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State) within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1eyj
Go back to
Magnesium binding site 2 out
of 2 in the Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State)
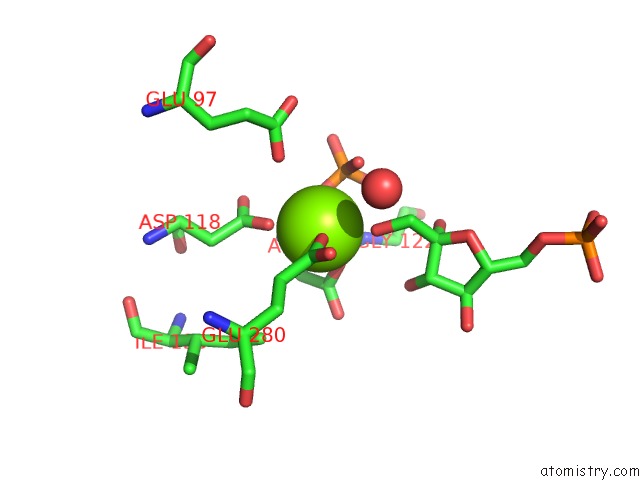
Mono view
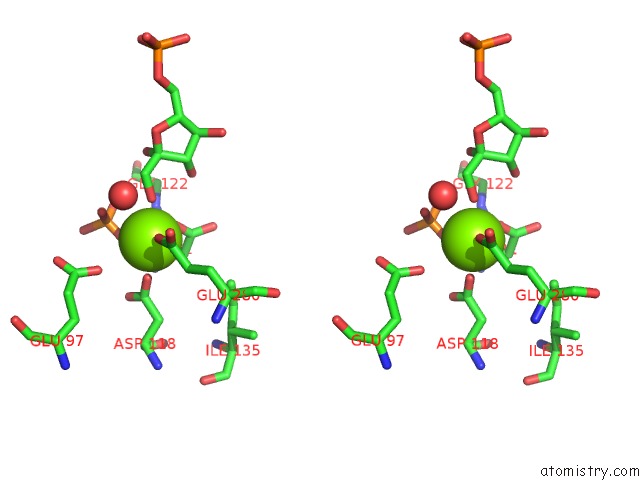
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Fructose-1,6-Bisphosphatase Complex with Amp, Magnesium, Fructose-6- Phosphate and Phosphate (T-State) within 5.0Å range:
|
Reference:
J.Y.Choe,
H.J.Fromm,
R.B.Honzatko.
Crystal Structures of Fructose 1,6-Bisphosphatase: Mechanism of Catalysis and Allosteric Inhibition Revealed in Product Complexes. Biochemistry V. 39 8565 2000.
ISSN: ISSN 0006-2960
PubMed: 10913263
DOI: 10.1021/BI000574G
Page generated: Tue Aug 13 03:04:31 2024
ISSN: ISSN 0006-2960
PubMed: 10913263
DOI: 10.1021/BI000574G
Last articles
F in 7LGXF in 7LGK
F in 7LG8
F in 7LD3
F in 7LCR
F in 7LCM
F in 7LCO
F in 7LCK
F in 7LCJ
F in 7LCI