Magnesium »
PDB 1gq9-1h7q »
1gs6 »
Magnesium in PDB 1gs6: Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase
Enzymatic activity of Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase
All present enzymatic activity of Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase:
1.7.2.1; 1.7.99.3;
1.7.2.1; 1.7.99.3;
Protein crystallography data
The structure of Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase, PDB code: 1gs6
was solved by
M.J.Ellis,
M.Prudencio,
F.E.Dodd,
R.W.Strange,
G.Sawers,
R.R.Eady,
S.S.Hasnain,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 60.00 / 2.2 |
| Space group | P 63 |
| Cell size a, b, c (Å), α, β, γ (°) | 106.860, 106.860, 64.026, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 14.8 / 18.1 |
Other elements in 1gs6:
The structure of Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase also contains other interesting chemical elements:
| Copper | (Cu) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase
(pdb code 1gs6). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase, PDB code: 1gs6:
In total only one binding site of Magnesium was determined in the Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase, PDB code: 1gs6:
Magnesium binding site 1 out of 1 in 1gs6
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase
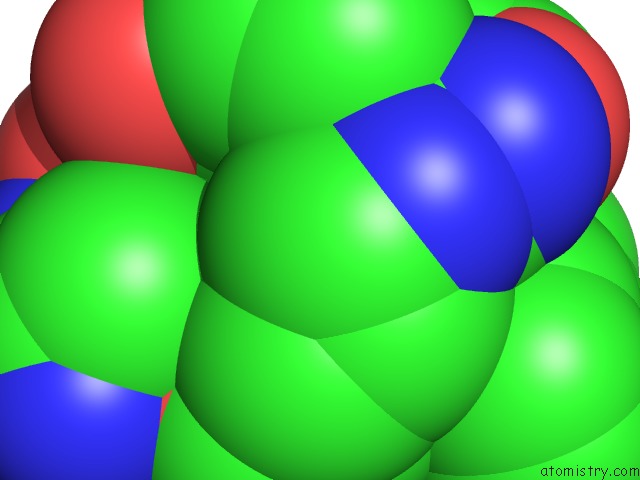
Mono view
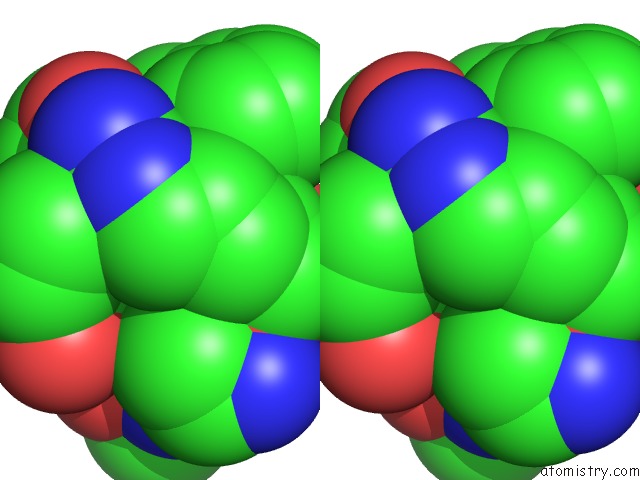
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of M144A Mutant of Alcaligenes Xylosoxidans Nitrite Reductase within 5.0Å range:
|
Reference:
M.J.Ellis,
M.Prudencio,
F.E.Dodd,
R.W.Strange,
G.Sawers,
R.R.Eady,
S.S.Hasnain.
Biochemical and Crystallographic Studies of the MET144ALA, ASP92ASN and HIS254PHE Mutants of the Nitrite Reductase From Alcaligenes Xylosoxidans Provide Insight Into the Enzyme Mechanism. J.Mol.Biol. V. 316 51 2002.
ISSN: ISSN 0022-2836
PubMed: 11829502
DOI: 10.1006/JMBI.2001.5304
Page generated: Sat Aug 9 21:23:04 2025
ISSN: ISSN 0022-2836
PubMed: 11829502
DOI: 10.1006/JMBI.2001.5304
Last articles
Mg in 3IJRMg in 3ILD
Mg in 3IKI
Mg in 3IKE
Mg in 3IKC
Mg in 3IIN
Mg in 3IJY
Mg in 3IJW
Mg in 3IJS
Mg in 3IJQ