Magnesium »
PDB 1iah-1iru »
1ijj »
Magnesium in PDB 1ijj: The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution
Protein crystallography data
The structure of The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution, PDB code: 1ijj
was solved by
S.M.Vorobiev,
M.R.Bubb,
S.C.Almo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 15.00 / 2.85 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 100.304, 102.183, 124.218, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23.3 / 30.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution
(pdb code 1ijj). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution, PDB code: 1ijj:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution, PDB code: 1ijj:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1ijj
Go back to
Magnesium binding site 1 out
of 2 in the The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution
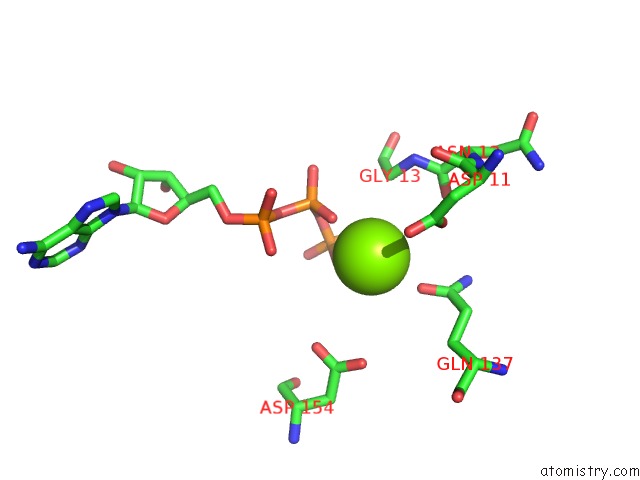
Mono view
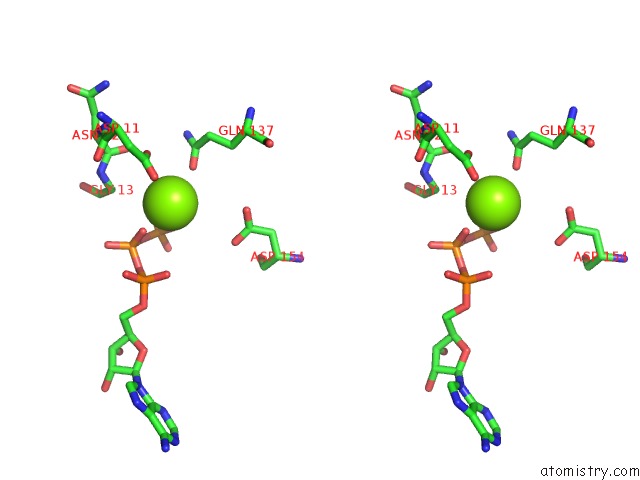
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1ijj
Go back to
Magnesium binding site 2 out
of 2 in the The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution
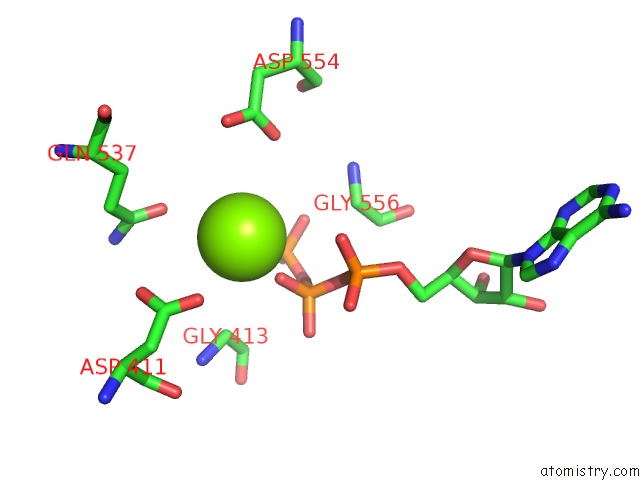
Mono view
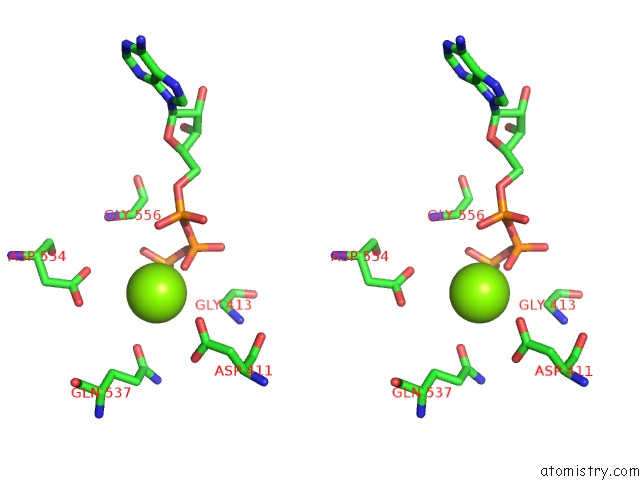
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of The X-Ray Crystal Structure of the Complex Between Rabbit Skeletal Muscle Actin and Latrunculin A at 2.85 A Resolution within 5.0Å range:
|
Reference:
M.R.Bubb,
L.Govindasamy,
E.G.Yarmola,
S.M.Vorobiev,
S.C.Almo,
T.Somasundaram,
M.S.Chapman,
M.Agbandje-Mckenna,
R.Mckenna.
Polylysine Induces An Antiparallel Actin Dimer That Nucleates Filament Assembly: Crystal Structure at 3.5-A Resolution J.Biol.Chem. V. 277 20999 2002.
ISSN: ISSN 0021-9258
PubMed: 11932258
DOI: 10.1074/JBC.M201371200
Page generated: Sat Aug 9 22:21:57 2025
ISSN: ISSN 0021-9258
PubMed: 11932258
DOI: 10.1074/JBC.M201371200
Last articles
Mg in 3I5XMg in 3I4K
Mg in 3I5F
Mg in 3I5C
Mg in 3I4N
Mg in 3I3E
Mg in 3I4D
Mg in 3I4M
Mg in 3I3D
Mg in 3I3B