Magnesium »
PDB 1jcg-1jwy »
1jh0 »
Magnesium in PDB 1jh0: Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
Protein crystallography data
The structure of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu, PDB code: 1jh0
was solved by
A.Camara-Artigas,
C.L.Magee,
J.C.Williams,
J.P.Allen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.90 / 3.50 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 141.800, 141.800, 187.400, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 22.5 / 26.9 |
Other elements in 1jh0:
The structure of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu also contains other interesting chemical elements:
| Iron | (Fe) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
(pdb code 1jh0). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu, PDB code: 1jh0:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu, PDB code: 1jh0:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 1jh0
Go back to
Magnesium binding site 1 out
of 4 in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
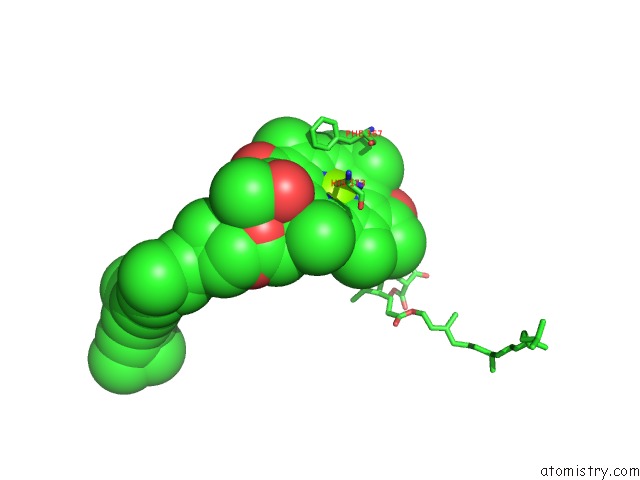
Mono view
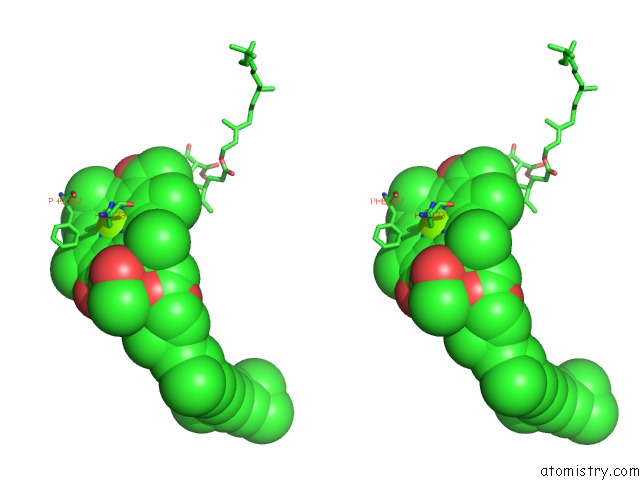
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 1jh0
Go back to
Magnesium binding site 2 out
of 4 in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
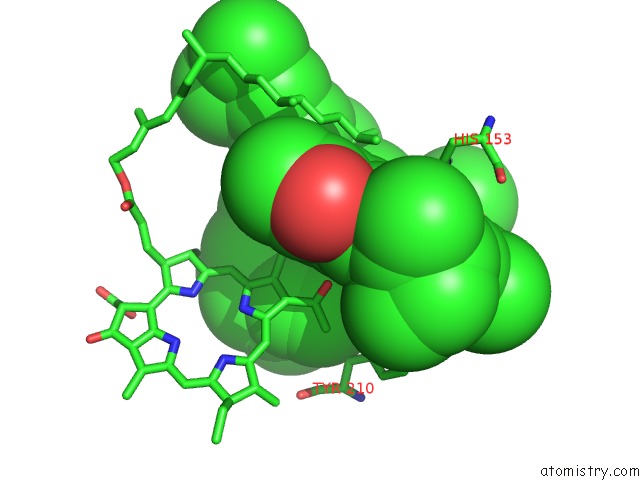
Mono view
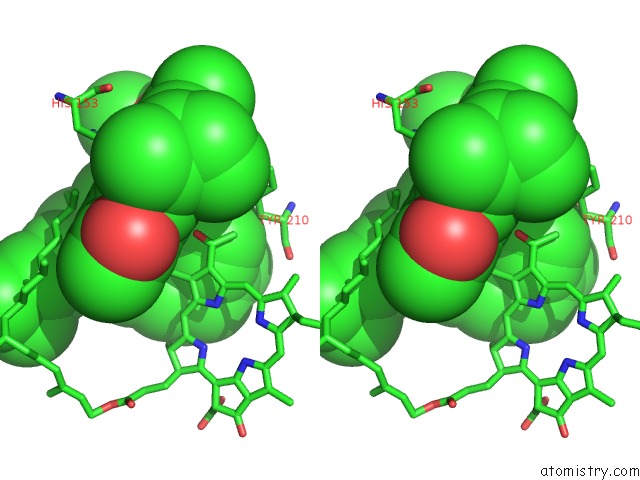
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 1jh0
Go back to
Magnesium binding site 3 out
of 4 in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
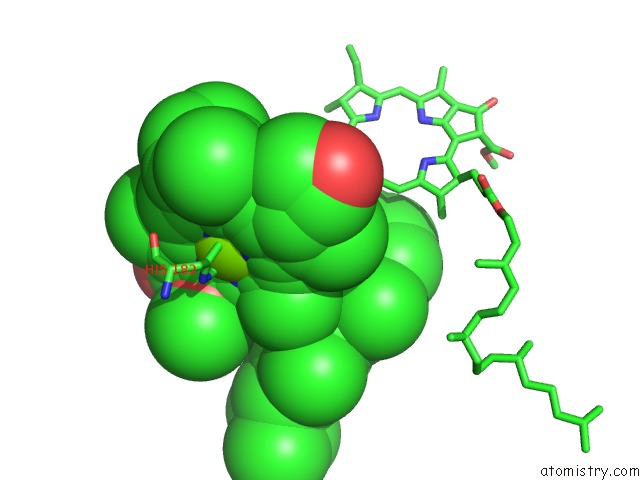
Mono view
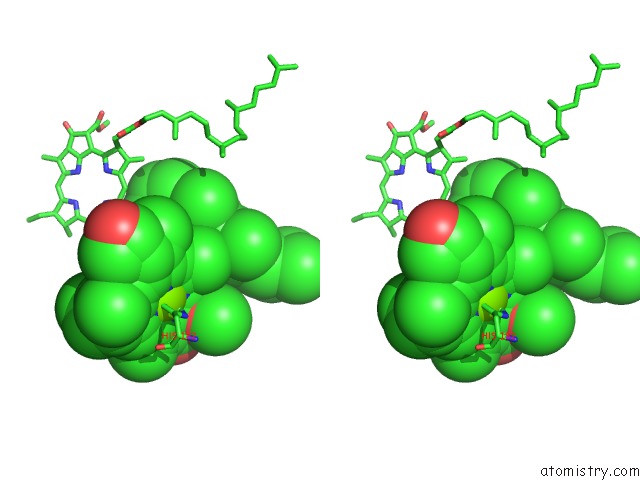
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 1jh0
Go back to
Magnesium binding site 4 out
of 4 in the Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu
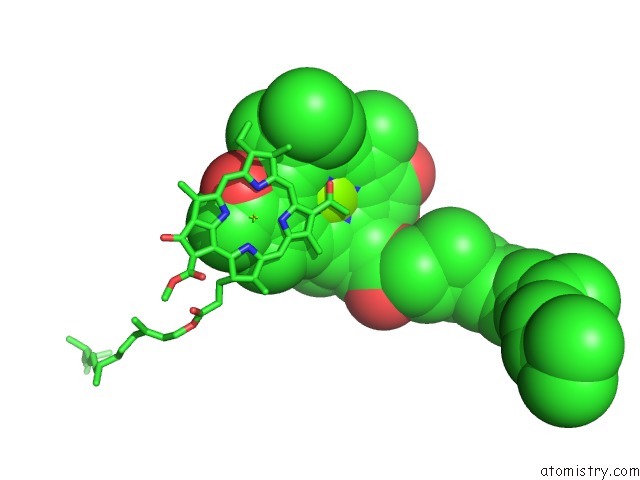
Mono view
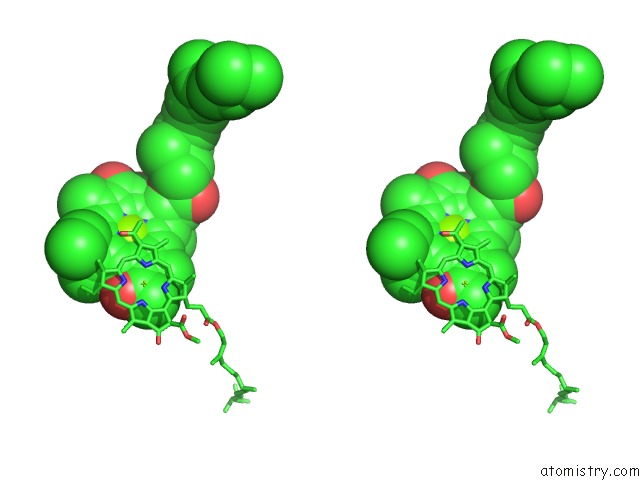
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Photosynthetic Reaction Center Mutant with Glu L 205 Replaced to Leu within 5.0Å range:
|
Reference:
A.Camara-Artigas,
C.L.Magee,
J.C.Williams,
J.P.Allen.
Individual Interactions Influence the Crystalline Order For Membrane Proteins. Acta Crystallogr.,Sect.D V. 57 1281 2001.
ISSN: ISSN 0907-4449
PubMed: 11526320
DOI: 10.1107/S090744490101109X
Page generated: Tue Aug 13 06:35:25 2024
ISSN: ISSN 0907-4449
PubMed: 11526320
DOI: 10.1107/S090744490101109X
Last articles
Cl in 5WLGCl in 5WL5
Cl in 5WKX
Cl in 5WKY
Cl in 5WL1
Cl in 5WHU
Cl in 5WKV
Cl in 5WKR
Cl in 5WKU
Cl in 5WKK