Magnesium »
PDB 1qc5-1qsh »
1qmz »
Magnesium in PDB 1qmz: Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex
Protein crystallography data
The structure of Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex, PDB code: 1qmz
was solved by
N.R.Brown,
M.E.M.Noble,
J.A.Endicott,
L.N.Johnson,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.0 / 2.20 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 152.600, 163.700, 73.300, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22 / 28 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex
(pdb code 1qmz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex, PDB code: 1qmz:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex, PDB code: 1qmz:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1qmz
Go back to
Magnesium binding site 1 out
of 2 in the Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex

Mono view
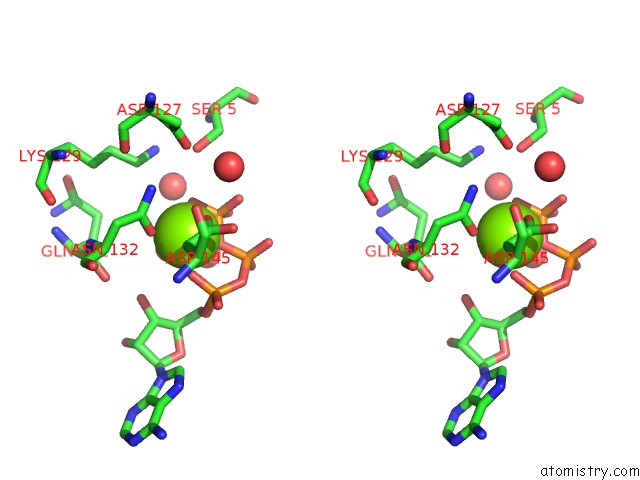
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1qmz
Go back to
Magnesium binding site 2 out
of 2 in the Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Phosphorylated CDK2-Cyclyin A-Substrate Peptide Complex within 5.0Å range:
|
Reference:
N.R.Brown,
M.E.Noble,
J.A.Endicott,
L.N.Johnson.
The Structural Basis For Specificity of Substrate and Recruitment Peptides For Cyclin-Dependent Kinases Nat.Cell Biol. V. 1 438 1999.
ISSN: ISSN 1465-7392
PubMed: 10559988
DOI: 10.1038/15674
Page generated: Tue Aug 13 11:53:43 2024
ISSN: ISSN 1465-7392
PubMed: 10559988
DOI: 10.1038/15674
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF