Magnesium »
PDB 1v5g-1vq4 »
1vcl »
Magnesium in PDB 1vcl: Crystal Structure of Hemolytic Lectin Cel-III
Protein crystallography data
The structure of Crystal Structure of Hemolytic Lectin Cel-III, PDB code: 1vcl
was solved by
T.Uchida,
T.Yamasaki,
S.Eto,
H.Sugawara,
G.Kurisu,
A.Nakagawa,
M.Kusunoki,
T.Hatakeyama,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 26.17 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 52.420, 65.370, 126.020, 90.00, 98.18, 90.00 |
| R / Rfree (%) | 16.5 / 20.1 |
Other elements in 1vcl:
The structure of Crystal Structure of Hemolytic Lectin Cel-III also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
| Calcium | (Ca) | 10 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Hemolytic Lectin Cel-III
(pdb code 1vcl). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Hemolytic Lectin Cel-III, PDB code: 1vcl:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Hemolytic Lectin Cel-III, PDB code: 1vcl:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 1vcl
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Hemolytic Lectin Cel-III
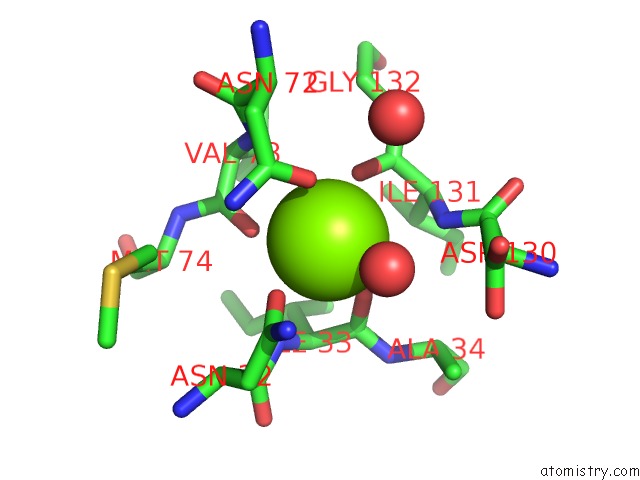
Mono view
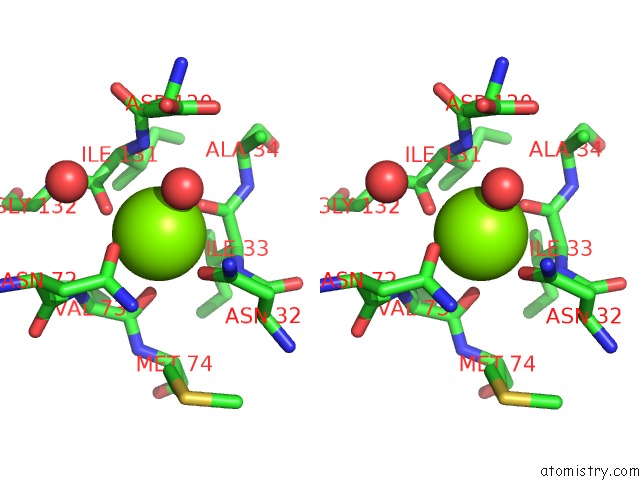
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Hemolytic Lectin Cel-III within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 1vcl
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Hemolytic Lectin Cel-III
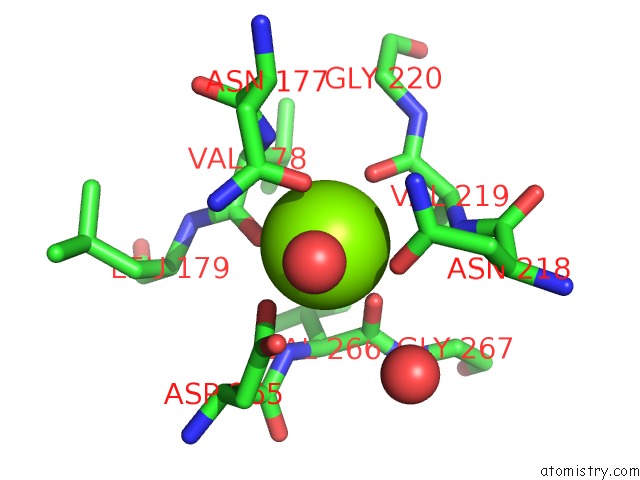
Mono view
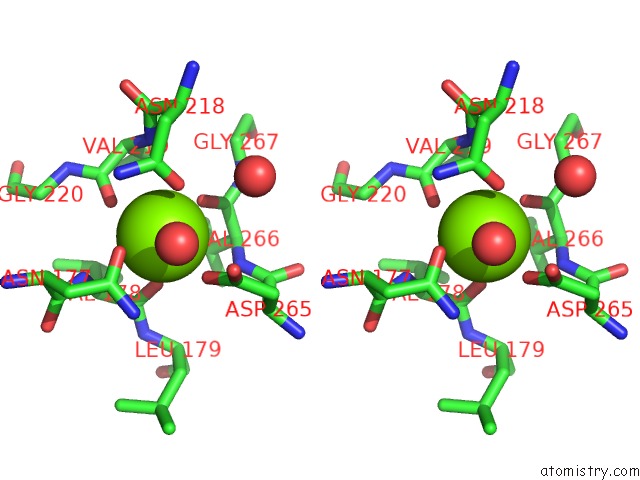
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Hemolytic Lectin Cel-III within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 1vcl
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Hemolytic Lectin Cel-III
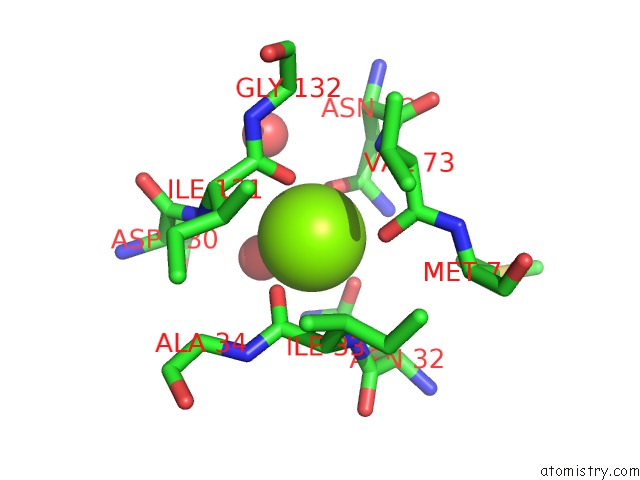
Mono view
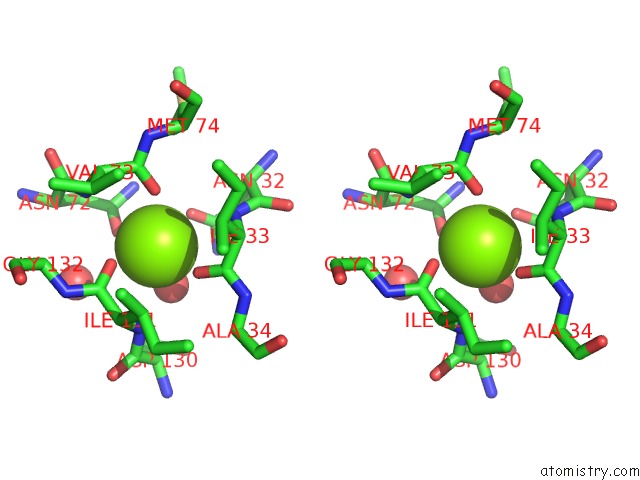
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Hemolytic Lectin Cel-III within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 1vcl
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Hemolytic Lectin Cel-III
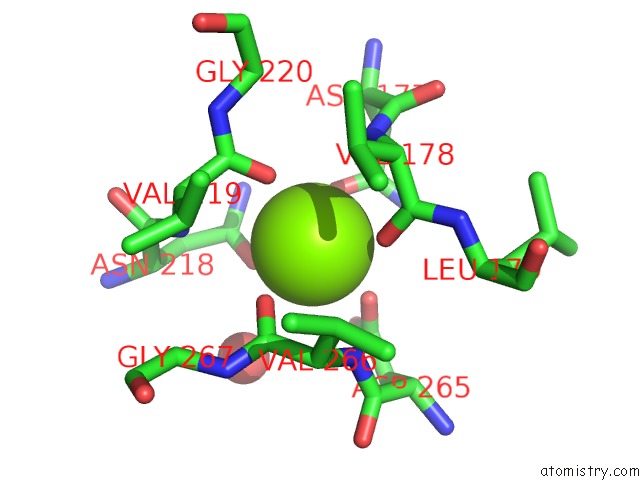
Mono view
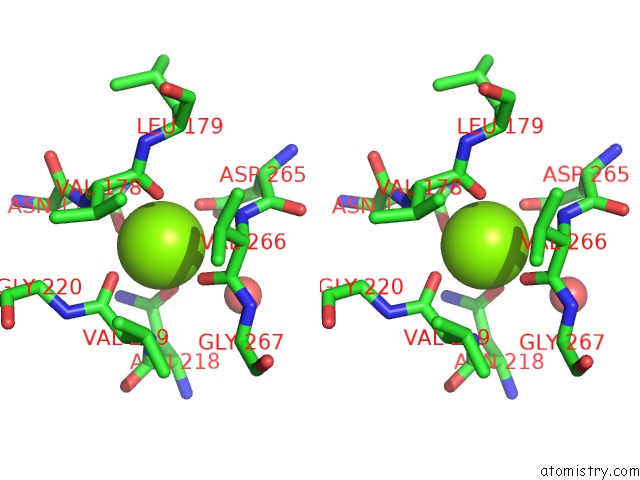
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Hemolytic Lectin Cel-III within 5.0Å range:
|
Reference:
T.Uchida,
T.Yamasaki,
S.Eto,
H.Sugawara,
G.Kurisu,
A.Nakagawa,
M.Kusunoki,
T.Hatakeyama.
Crystal Structure of the Hemolytic Lectin Cel-III Isolated From the Marine Invertebrate Cucumaria Echinata: Implications of Domain Structure For Its Membrane Pore-Formation Mechanism J.Biol.Chem. V. 279 37133 2004.
ISSN: ISSN 0021-9258
PubMed: 15194688
DOI: 10.1074/JBC.M404065200
Page generated: Tue Aug 13 15:02:47 2024
ISSN: ISSN 0021-9258
PubMed: 15194688
DOI: 10.1074/JBC.M404065200
Last articles
F in 4EEVF in 4E7P
F in 4EAD
F in 4E3N
F in 4E4X
F in 4E91
F in 4E5D
F in 4E1V
F in 4DLE
F in 4E28