Magnesium »
PDB 2a6h-2alz »
2akm »
Magnesium in PDB 2akm: Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
Enzymatic activity of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
All present enzymatic activity of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex:
4.2.1.11;
4.2.1.11;
Protein crystallography data
The structure of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex, PDB code: 2akm
was solved by
J.Qin,
G.Chai,
J.M.Brewer,
L.L.Lovelace,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.92 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 111.739, 119.692, 68.299, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.1 / 24.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
(pdb code 2akm). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex, PDB code: 2akm:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex, PDB code: 2akm:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 2akm
Go back to
Magnesium binding site 1 out
of 3 in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
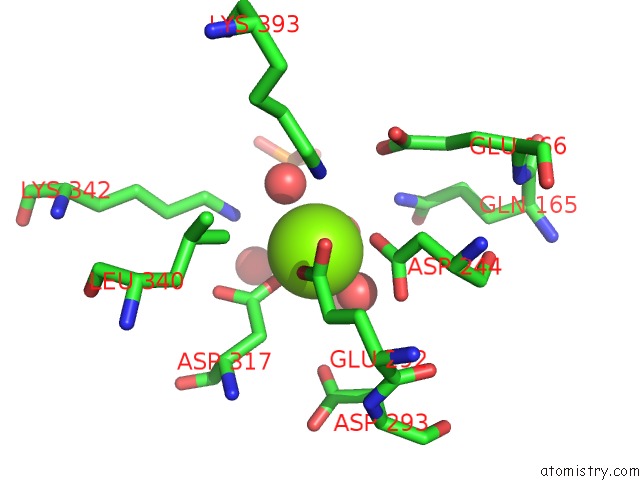
Mono view
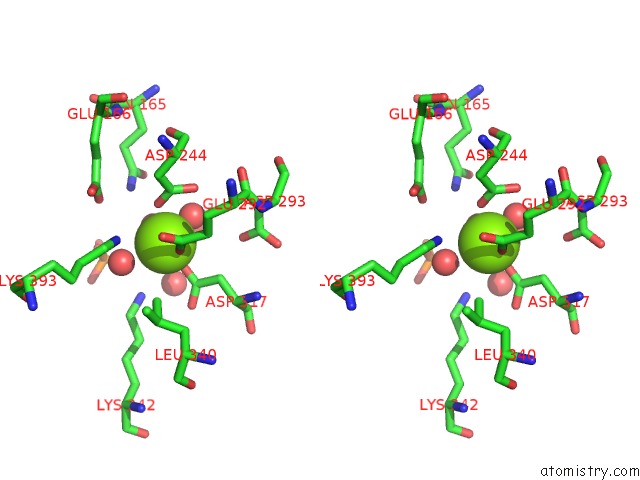
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 2akm
Go back to
Magnesium binding site 2 out
of 3 in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
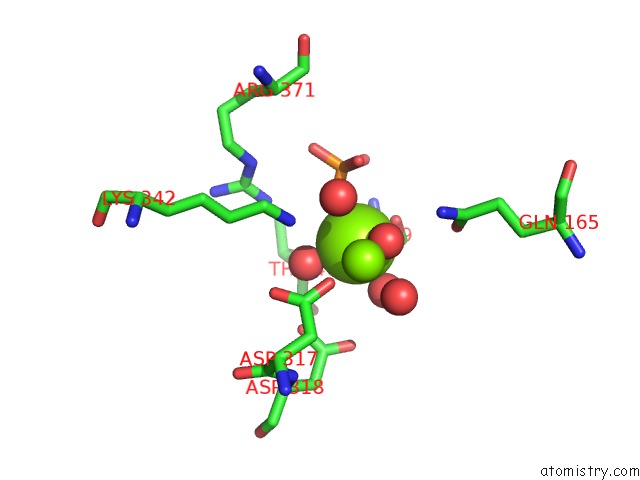
Mono view
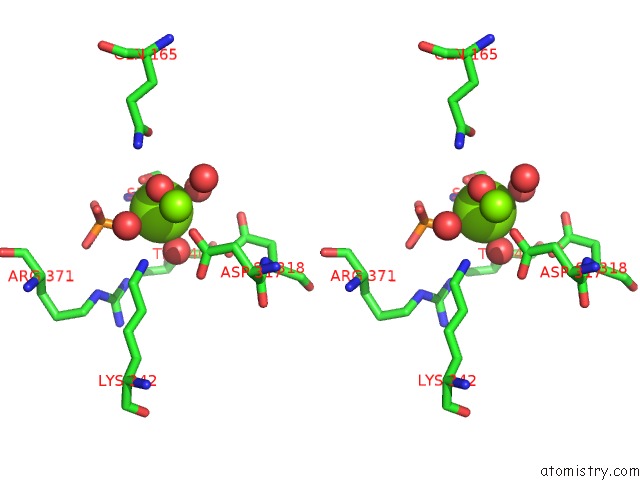
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 2akm
Go back to
Magnesium binding site 3 out
of 3 in the Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex
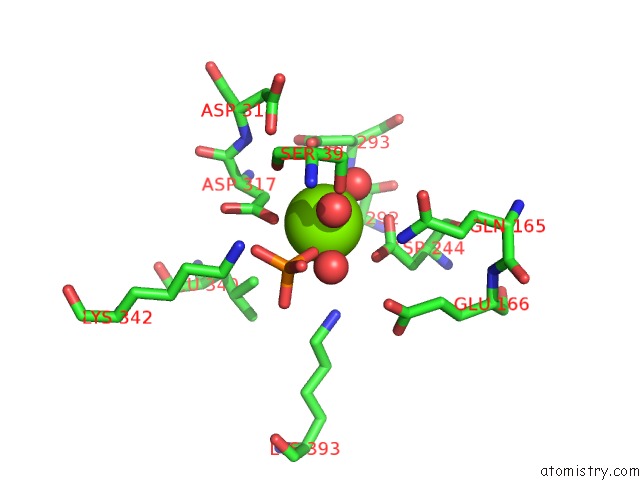
Mono view
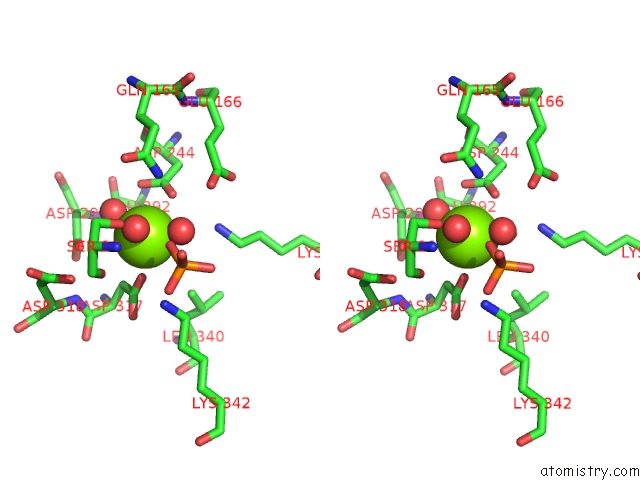
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Fluoride Inhibition of Enolase: Crystal Structure of the Inhibitory Complex within 5.0Å range:
|
Reference:
J.Qin,
G.Chai,
J.M.Brewer,
L.L.Lovelace,
L.Lebioda.
Fluoride Inhibition of Enolase: Crystal Structure and Thermodynamics Biochemistry V. 45 793 2006.
ISSN: ISSN 0006-2960
PubMed: 16411755
DOI: 10.1021/BI051558S
Page generated: Sun Aug 10 09:47:18 2025
ISSN: ISSN 0006-2960
PubMed: 16411755
DOI: 10.1021/BI051558S
Last articles
Mg in 5SHRMg in 5SHQ
Mg in 5SHP
Mg in 5SHN
Mg in 5SHO
Mg in 5SHL
Mg in 5SHM
Mg in 5SHK
Mg in 5SHJ
Mg in 5SHI