Magnesium »
PDB 2bhd-2bt1 »
2bif »
Magnesium in PDB 2bif: 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site
Enzymatic activity of 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site
All present enzymatic activity of 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site:
2.7.1.105; 3.1.3.46;
2.7.1.105; 3.1.3.46;
Protein crystallography data
The structure of 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site, PDB code: 2bif
was solved by
M.H.Yuen,
C.A.Hasemann,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.40 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 61.740, 73.510, 76.700, 116.90, 99.31, 105.20 |
| R / Rfree (%) | 20 / 24.4 |
Magnesium Binding Sites:
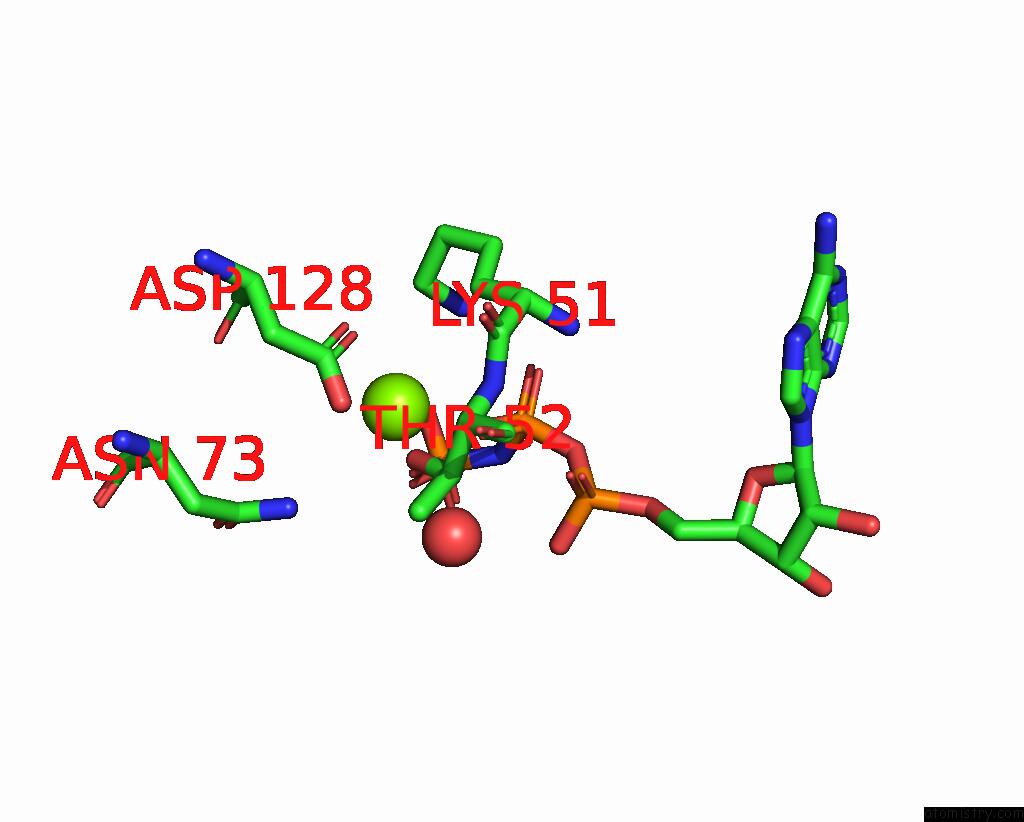
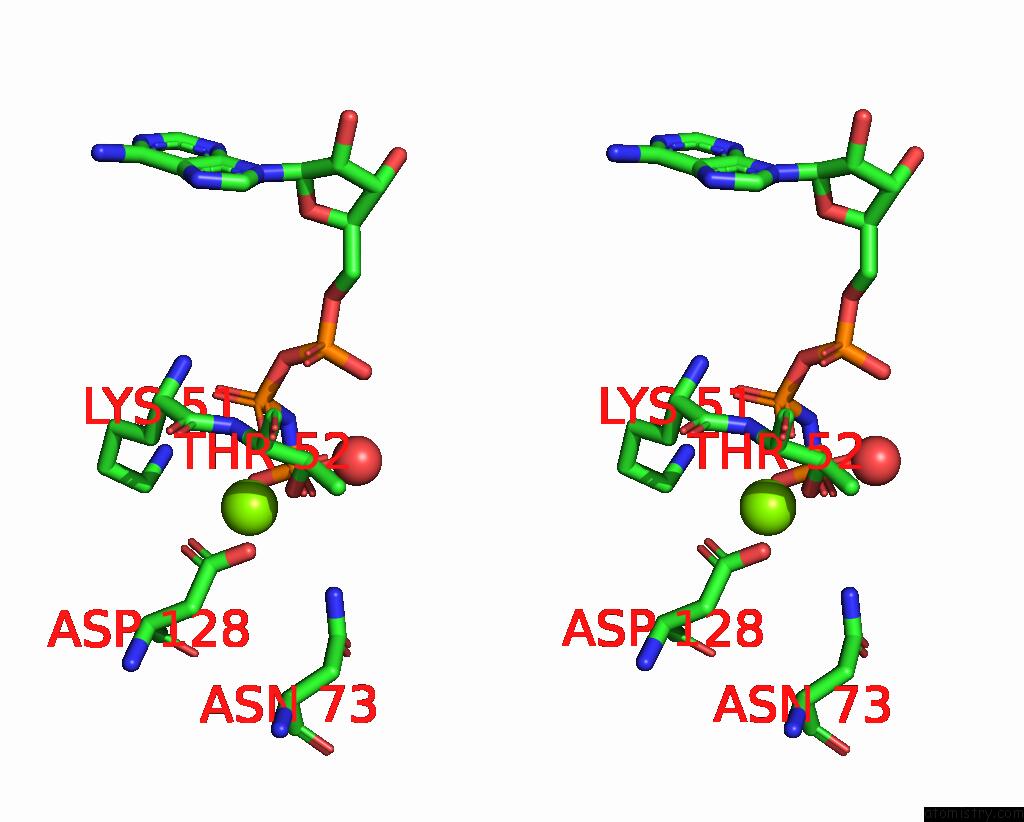
The binding sites of Magnesium atom in the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site
(pdb code 2bif). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site, PDB code: 2bif:
In total only one binding site of Magnesium was determined in the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site, PDB code: 2bif:
Magnesium binding site 1 out of 1 in 2bif
Go back to
Magnesium binding site 1 out
of 1 in the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase H256A Mutant with F6P in Phosphatase Active Site within 5.0Å range:
|
Reference:
M.H.Yuen,
H.Mizuguchi,
Y.H.Lee,
P.F.Cook,
K.Uyeda,
C.A.Hasemann.
Crystal Structure of the H256A Mutant of Rat Testis Fructose-6-Phosphate,2-Kinase/Fructose-2,6- Bisphosphatase. Fructose 6-Phosphate in the Active Site Leads to Mechanisms For Both Mutant and Wild Type Bisphosphatase Activities. J.Biol.Chem. V. 274 2176 1999.
ISSN: ISSN 0021-9258
PubMed: 9890980
DOI: 10.1074/JBC.274.4.2176
Page generated: Sun Aug 10 10:02:01 2025
ISSN: ISSN 0021-9258
PubMed: 9890980
DOI: 10.1074/JBC.274.4.2176
Last articles
Mg in 6CUUMg in 6CUO
Mg in 6CUR
Mg in 6CUP
Mg in 6CU6
Mg in 6CU2
Mg in 6CU1
Mg in 6CTZ
Mg in 6CTX
Mg in 6CTW