Magnesium »
PDB 2gqr-2haw »
2gt4 »
Magnesium in PDB 2gt4: Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2
Protein crystallography data
The structure of Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2, PDB code: 2gt4
was solved by
S.B.Gabelli,
M.A.Bianchet,
H.F.Azurmendi,
A.S.Mildvan,
L.A.Amzel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 77.61 / 2.30 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 137.785, 93.893, 66.103, 90.00, 91.23, 90.00 |
| R / Rfree (%) | 18.6 / 22.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2
(pdb code 2gt4). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2, PDB code: 2gt4:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2, PDB code: 2gt4:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 2gt4
Go back to
Magnesium binding site 1 out
of 3 in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2
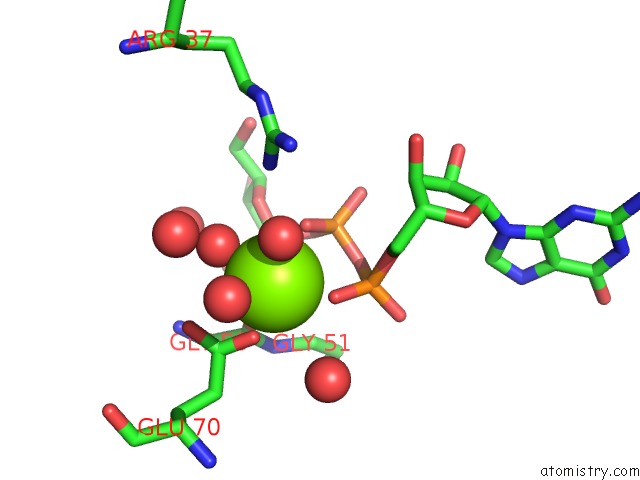
Mono view
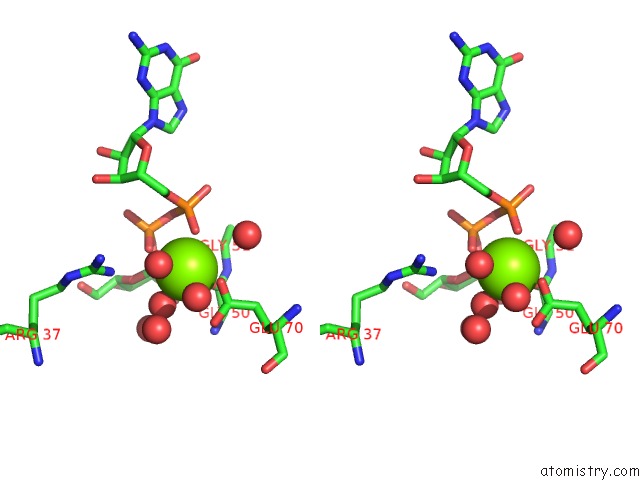
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2 within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 2gt4
Go back to
Magnesium binding site 2 out
of 3 in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2
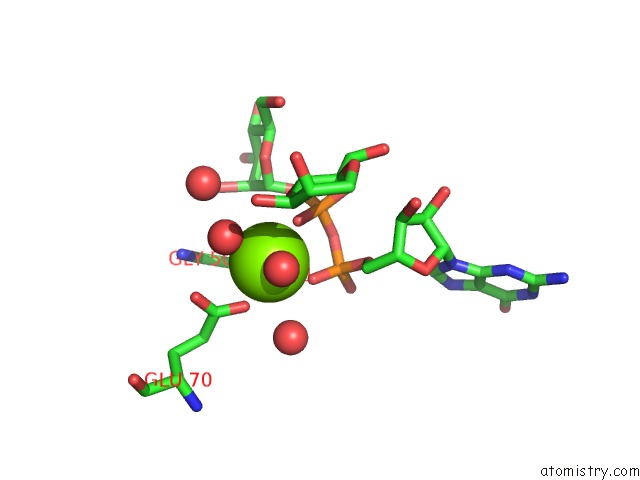
Mono view
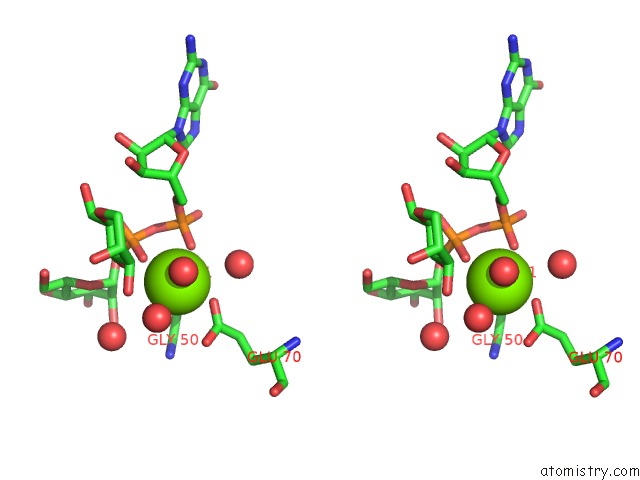
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2 within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 2gt4
Go back to
Magnesium binding site 3 out
of 3 in the Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2
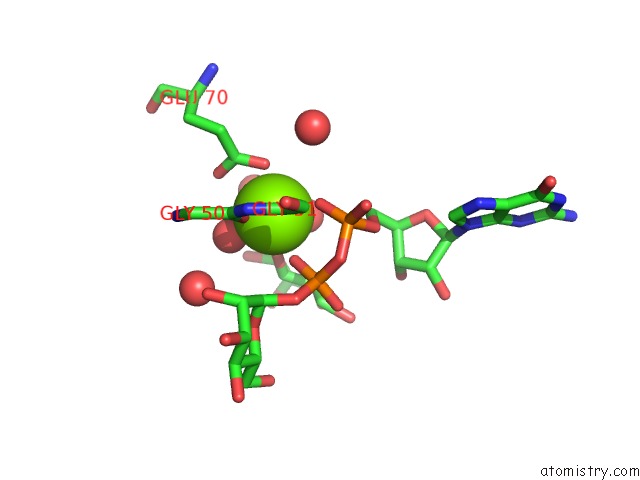
Mono view
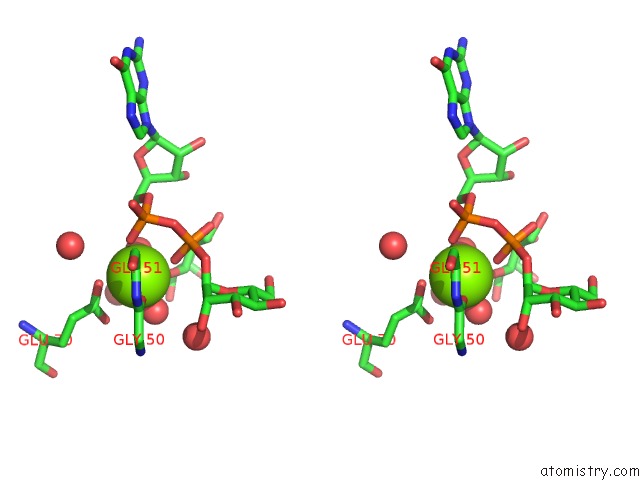
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of the Y103F Mutant of the Gdp-Mannose Mannosyl Hydrolase in Complex with Gdp-Mannose and Mg+2 within 5.0Å range:
|
Reference:
S.B.Gabelli,
H.F.Azurmendi,
M.A.Bianchet,
L.M.Amzel,
A.S.Mildvan.
X-Ray, uc(Nmr), and Mutational Studies of the Catalytic Cycle of the Gdp-Mannose Mannosyl Hydrolase Reaction. Biochemistry V. 45 11290 2006.
ISSN: ISSN 0006-2960
PubMed: 16981689
DOI: 10.1021/BI061239G
Page generated: Tue Aug 13 23:35:42 2024
ISSN: ISSN 0006-2960
PubMed: 16981689
DOI: 10.1021/BI061239G
Last articles
F in 4IBJF in 4IFY
F in 4IFV
F in 4IDO
F in 4ICC
F in 4IBI
F in 4IAH
F in 4IAE
F in 4I9H
F in 4I9N