Magnesium »
PDB 2hb4-2hkj »
2hjp »
Magnesium in PDB 2hjp: Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++
Protein crystallography data
The structure of Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++, PDB code: 2hjp
was solved by
C.C.H.Chen,
O.Herzberg,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.80 / 1.90 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 74.890, 79.180, 93.430, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.9 / 22.5 |
Other elements in 2hjp:
The structure of Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++ also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
| Sodium | (Na) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++
(pdb code 2hjp). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++, PDB code: 2hjp:
In total only one binding site of Magnesium was determined in the Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++, PDB code: 2hjp:
Magnesium binding site 1 out of 1 in 2hjp
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++
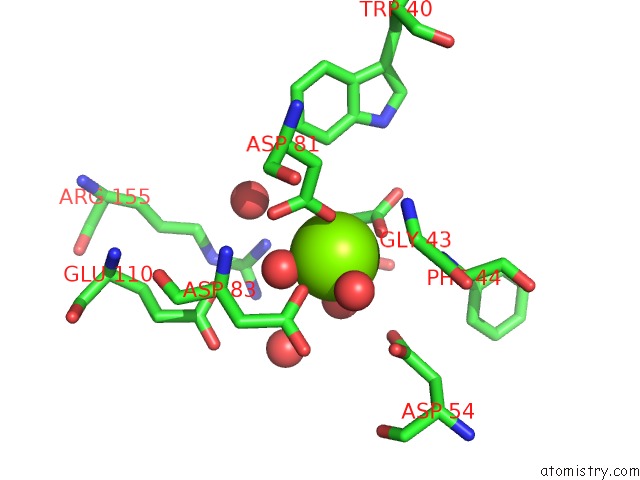
Mono view
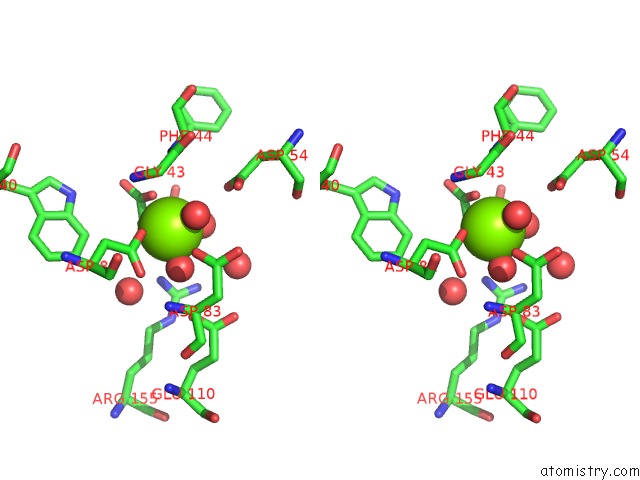
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Phosphonopyruvate Hydrolase Complex with Phosphonopyruvate and Mg++ within 5.0Å range:
|
Reference:
C.C.H.Chen,
Y.Han,
W.Niu,
A.N.Kulakova,
A.Howard,
J.P.Quinn,
D.Dunaway-Mariano,
O.Herzberg.
Structure and Kinetics of Phosphonopyruvate Hydrolase From Voriovorax Sp. PAL2: New Insight Into the Divergence of Catalysis Within the Pep Mutase/Isocitrate Lyase Superfamily Biochemistry V. 45 11491 2006.
ISSN: ISSN 0006-2960
PubMed: 16981709
DOI: 10.1021/BI061208L
Page generated: Tue Aug 13 23:53:47 2024
ISSN: ISSN 0006-2960
PubMed: 16981709
DOI: 10.1021/BI061208L
Last articles
Ca in 5UWLCa in 5UVE
Ca in 5UW5
Ca in 5UW6
Ca in 5UVL
Ca in 5UVG
Ca in 5UUE
Ca in 5UUY
Ca in 5UUH
Ca in 5UUG