Magnesium »
PDB 2hld-2hxf »
2hmf »
Magnesium in PDB 2hmf: Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
Enzymatic activity of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
All present enzymatic activity of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate:
2.7.2.4;
2.7.2.4;
Protein crystallography data
The structure of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate, PDB code: 2hmf
was solved by
C.R.Faehnle,
R.E.Viola,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 2.70 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 101.741, 104.508, 192.920, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 24.1 / 27.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
(pdb code 2hmf). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate, PDB code: 2hmf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate, PDB code: 2hmf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 2hmf
Go back to
Magnesium binding site 1 out
of 4 in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
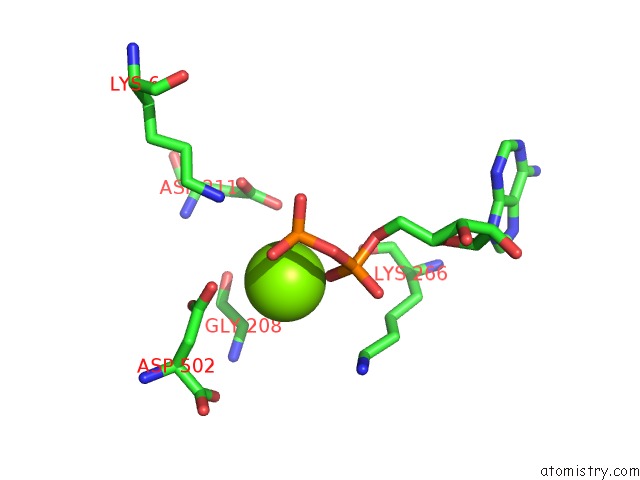
Mono view
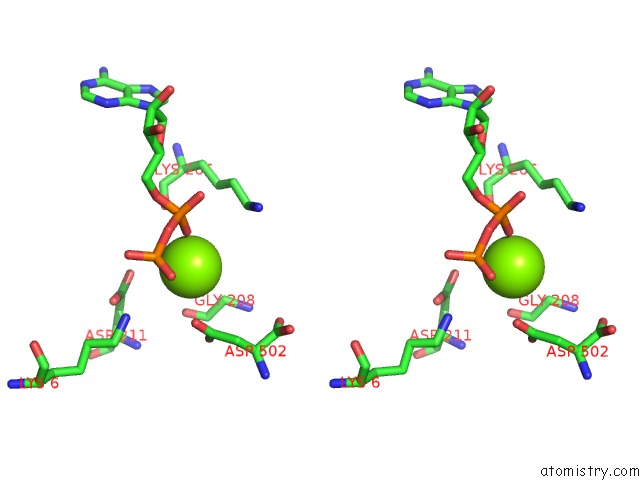
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 2hmf
Go back to
Magnesium binding site 2 out
of 4 in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
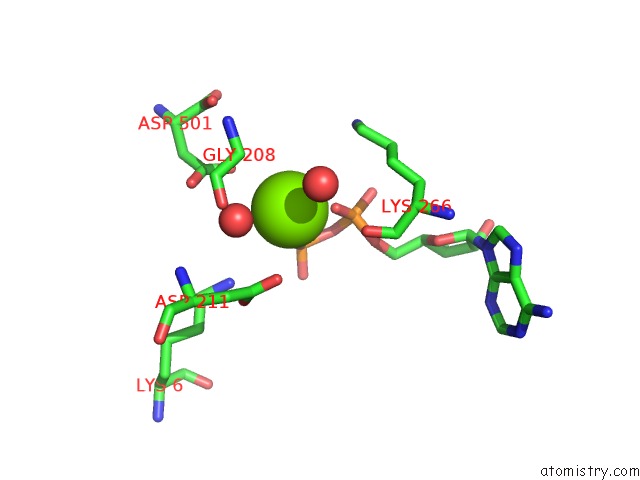
Mono view
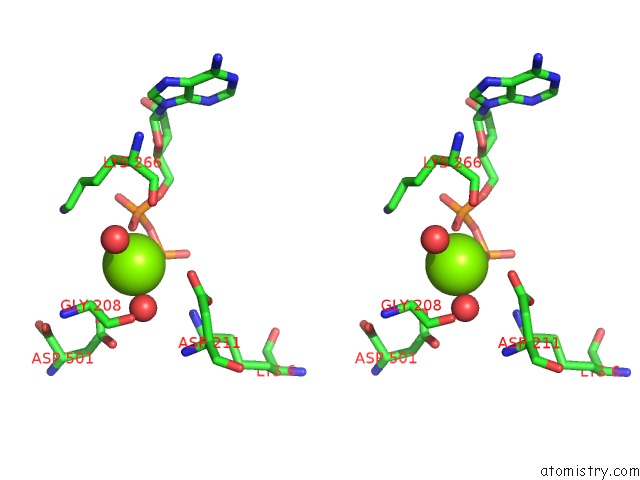
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 2hmf
Go back to
Magnesium binding site 3 out
of 4 in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
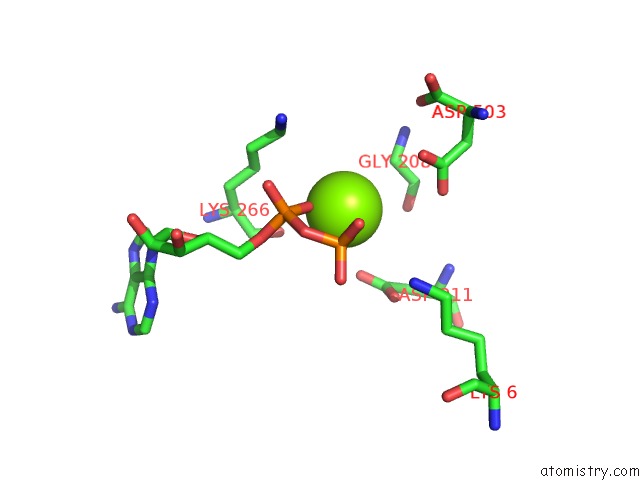
Mono view
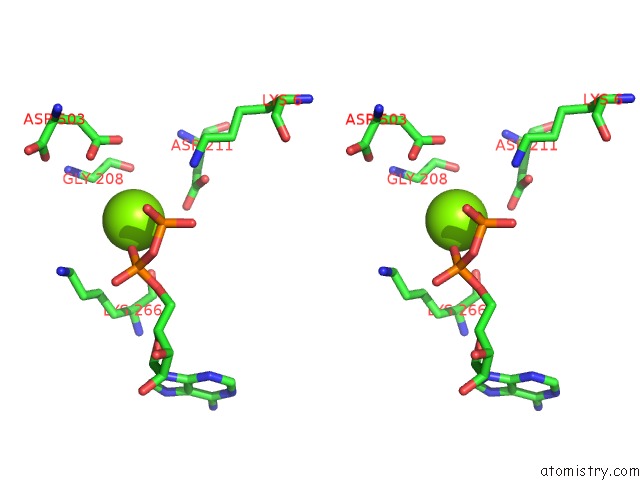
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 2hmf
Go back to
Magnesium binding site 4 out
of 4 in the Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate
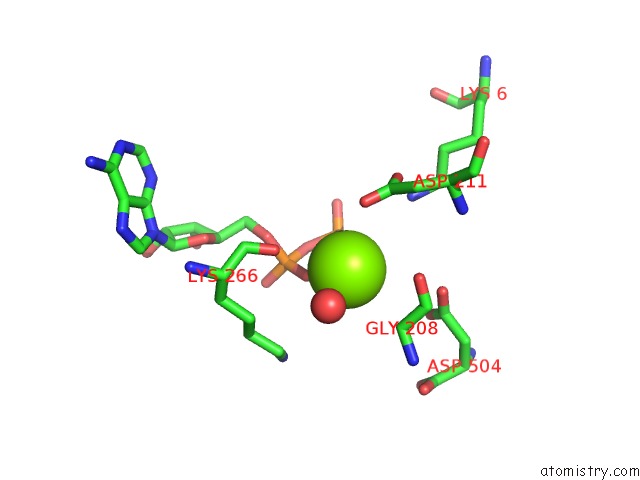
Mono view
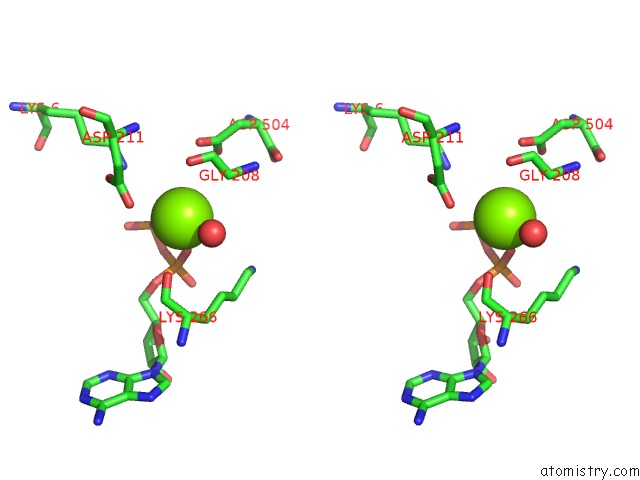
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of A Threonine Sensitive Aspartokinase From Methanococcus Jannaschii Complexed with Mg-Adp and Aspartate within 5.0Å range:
|
Reference:
C.R.Faehnle,
X.Liu,
A.Pavlovsky,
R.E.Viola.
The Initial Step in the Archaeal Aspartate Biosynthetic Pathway Catalyzed By A Monofunctional Aspartokinase. Acta Crystallogr.,Sect.F V. 62 962 2006.
ISSN: ESSN 1744-3091
PubMed: 17012784
DOI: 10.1107/S1744309106038279
Page generated: Sun Aug 10 11:24:27 2025
ISSN: ESSN 1744-3091
PubMed: 17012784
DOI: 10.1107/S1744309106038279
Last articles
Mg in 6DW4Mg in 6DW3
Mg in 6DUQ
Mg in 6DVK
Mg in 6DUK
Mg in 6DV9
Mg in 6DUS
Mg in 6DUH
Mg in 6DUG
Mg in 6DUF