Magnesium »
PDB 2mtk-2o52 »
2nz4 »
Magnesium in PDB 2nz4: Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
Protein crystallography data
The structure of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor, PDB code: 2nz4
was solved by
J.C.Cochrane,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 34.94 / 2.50 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.127, 234.157, 105.003, 90.00, 90.65, 90.00 |
| R / Rfree (%) | 22.2 / 26.9 |
Magnesium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Magnesium atom in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor (pdb code 2nz4). This binding sites where shown within 5.0 Angstroms radius around Magnesium atom.In total 16 binding sites of Magnesium where determined in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor, PDB code: 2nz4:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Magnesium binding site 1 out of 16 in 2nz4
Go back to
Magnesium binding site 1 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
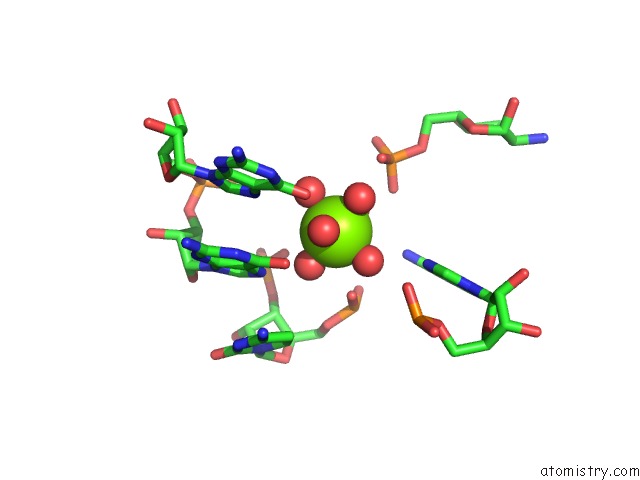
Mono view
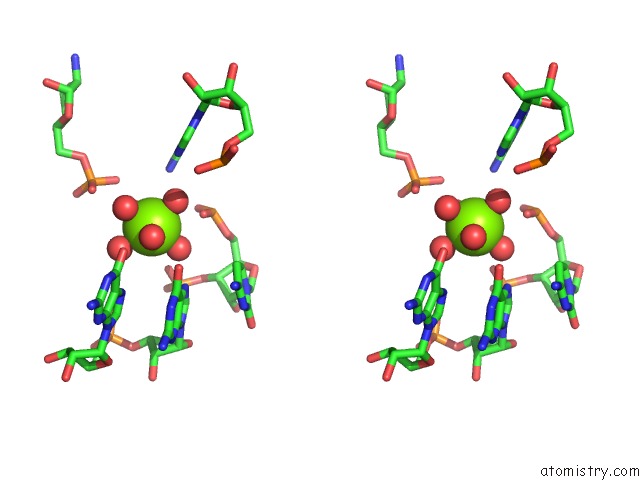
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 2 out of 16 in 2nz4
Go back to
Magnesium binding site 2 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
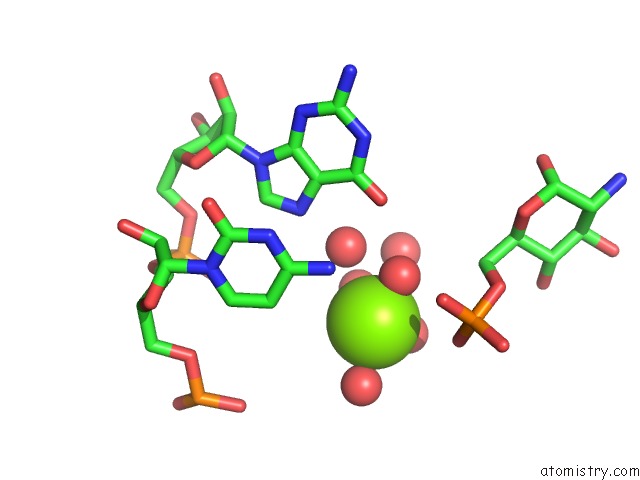
Mono view
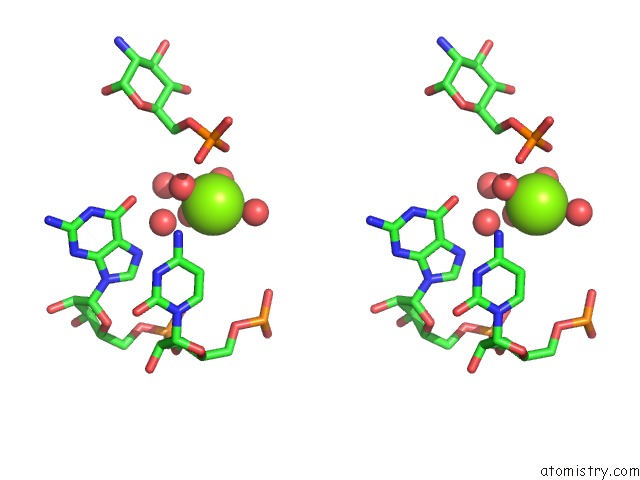
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 3 out of 16 in 2nz4
Go back to
Magnesium binding site 3 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
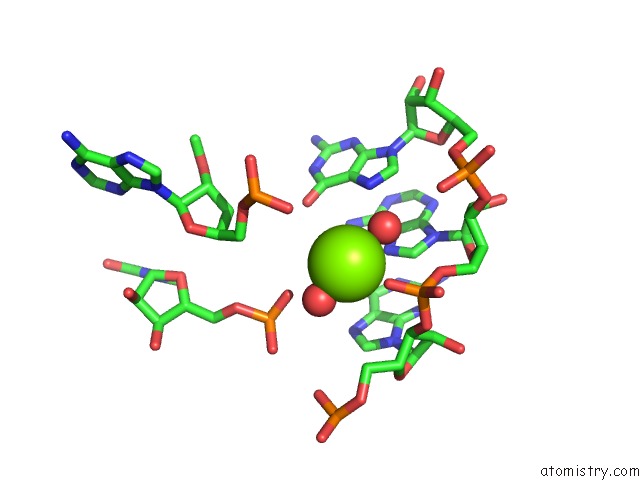
Mono view
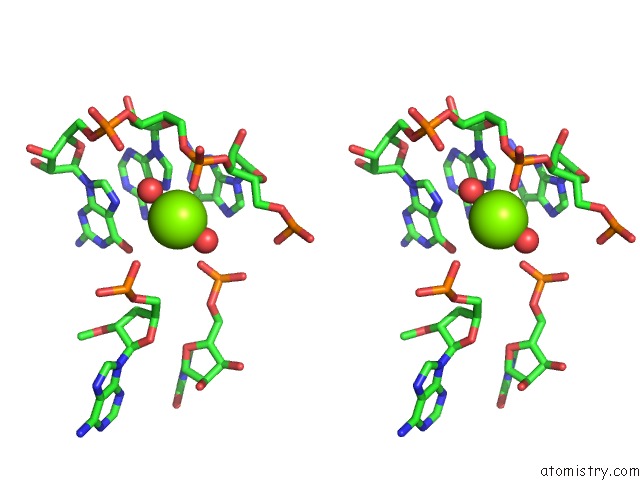
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 4 out of 16 in 2nz4
Go back to
Magnesium binding site 4 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
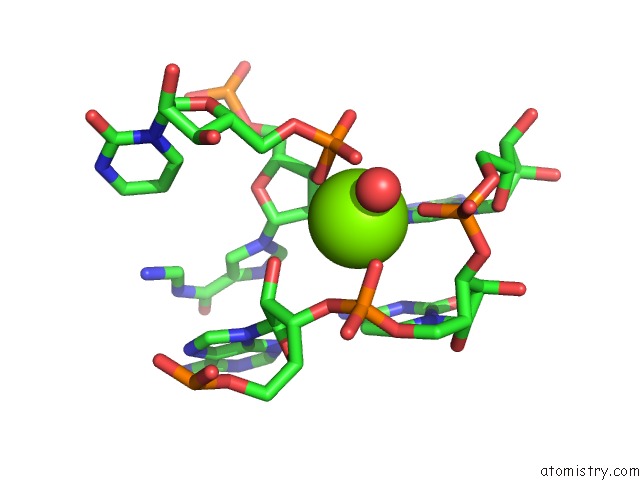
Mono view
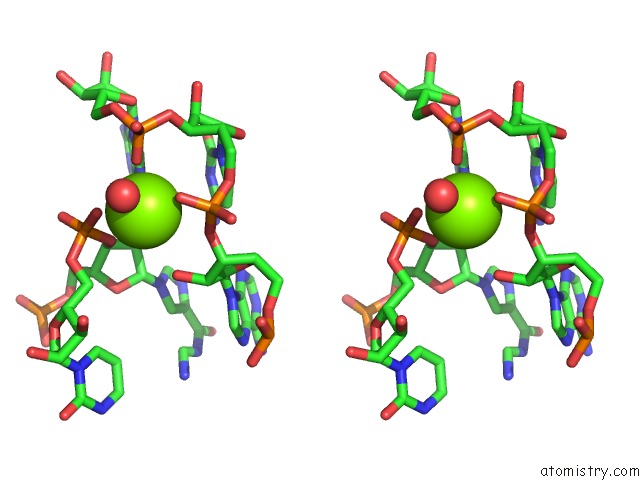
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 5 out of 16 in 2nz4
Go back to
Magnesium binding site 5 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
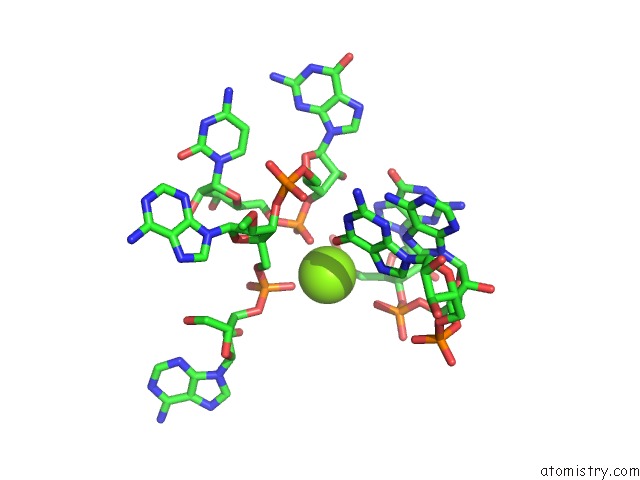
Mono view
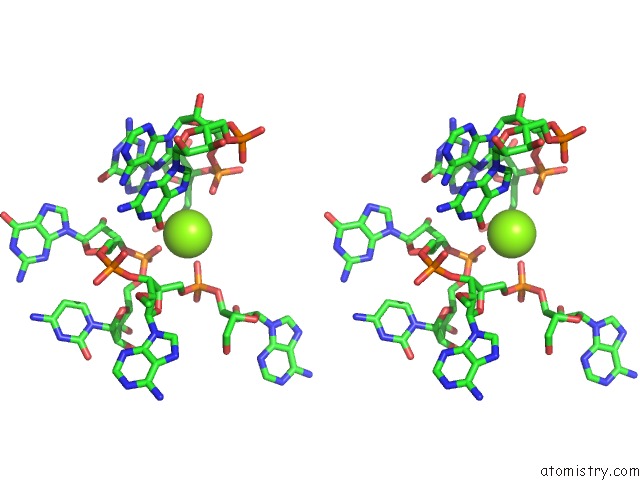
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 6 out of 16 in 2nz4
Go back to
Magnesium binding site 6 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
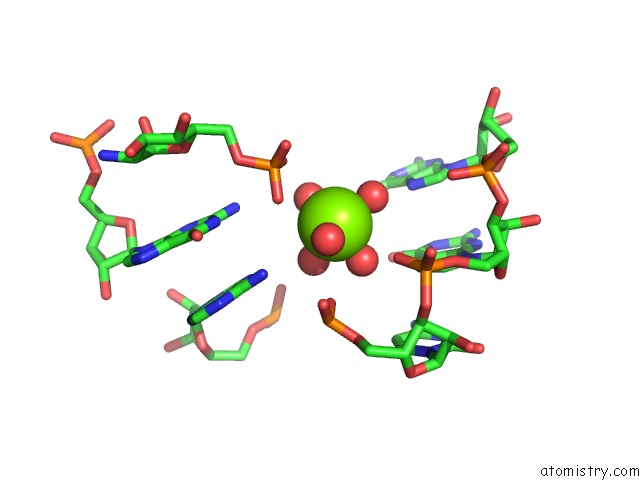
Mono view
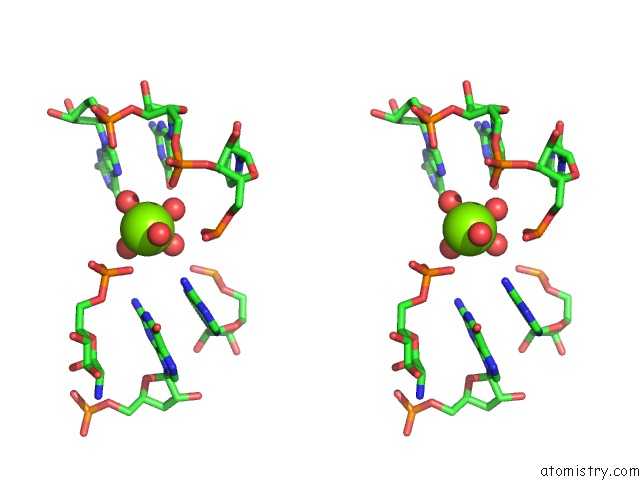
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 7 out of 16 in 2nz4
Go back to
Magnesium binding site 7 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
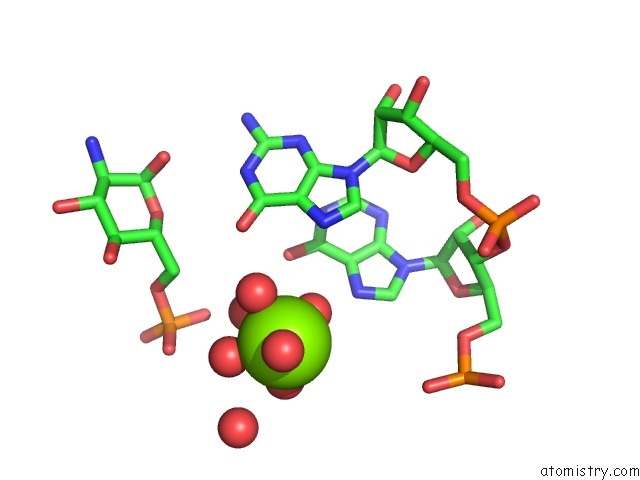
Mono view
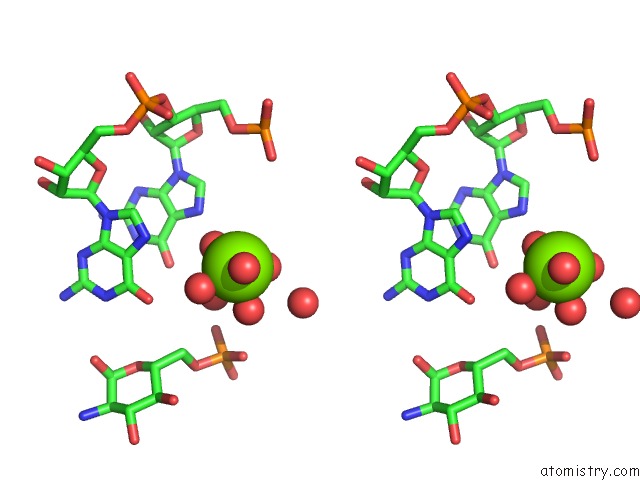
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 8 out of 16 in 2nz4
Go back to
Magnesium binding site 8 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
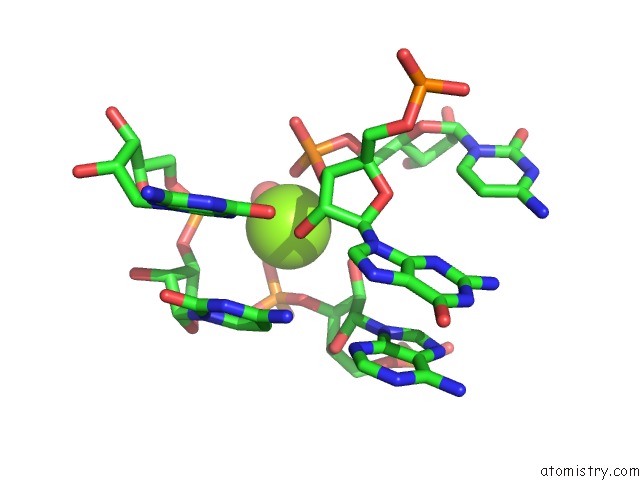
Mono view
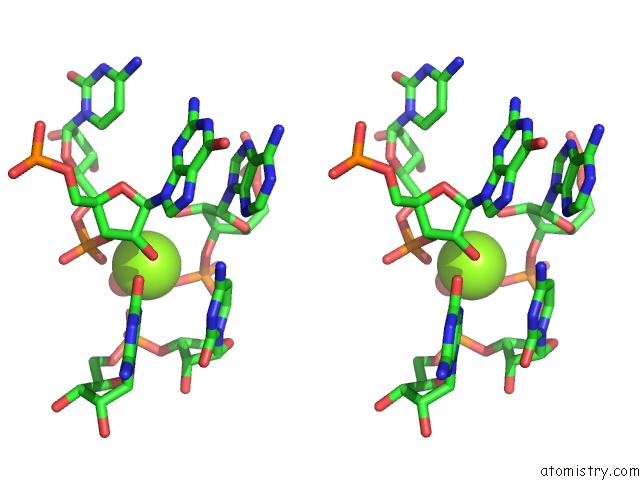
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 9 out of 16 in 2nz4
Go back to
Magnesium binding site 9 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
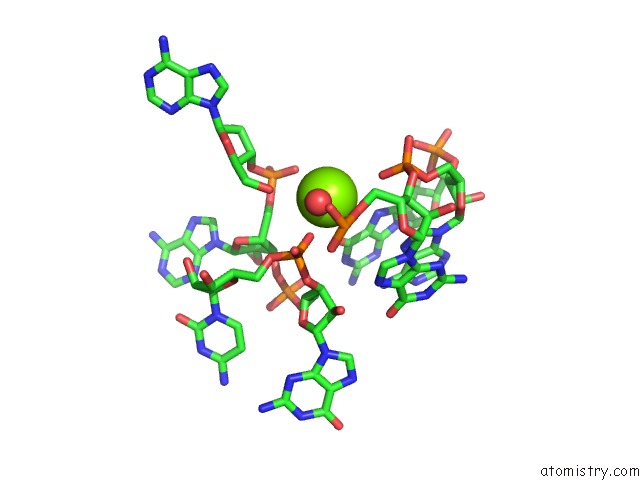
Mono view
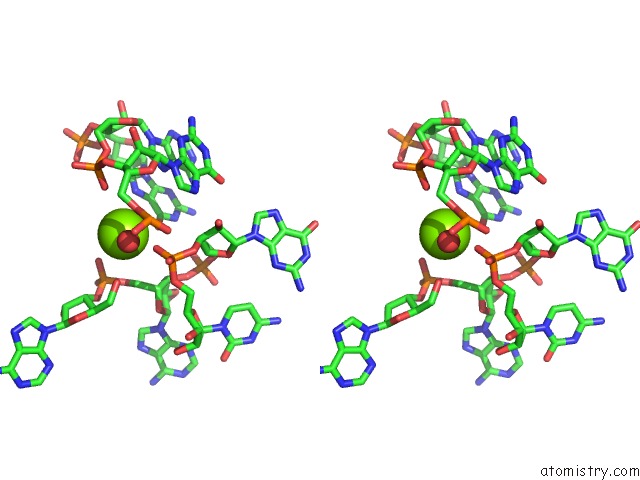
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Magnesium binding site 10 out of 16 in 2nz4
Go back to
Magnesium binding site 10 out
of 16 in the Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor
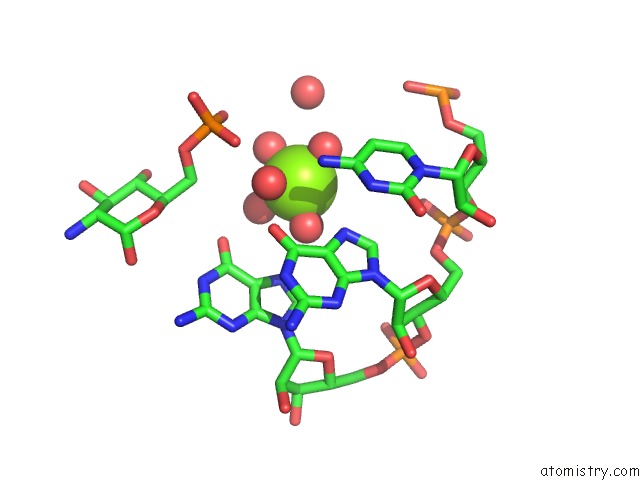
Mono view
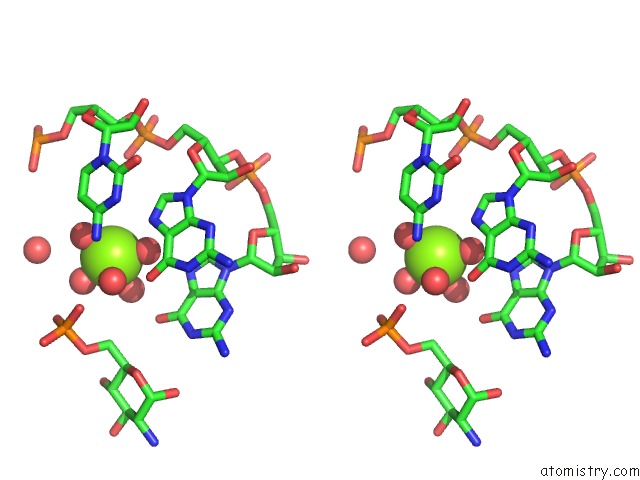
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 10 of Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor within 5.0Å range:
|
Reference:
J.C.Cochrane,
S.V.Lipchock,
S.A.Strobel.
Structural Investigation of the Glms Ribozyme Bound to Its Catalytic Cofactor Chem.Biol. V. 14 97 2007.
ISSN: ISSN 1074-5521
PubMed: 17196404
DOI: 10.1016/J.CHEMBIOL.2006.12.005
Page generated: Sun Aug 10 12:08:40 2025
ISSN: ISSN 1074-5521
PubMed: 17196404
DOI: 10.1016/J.CHEMBIOL.2006.12.005
Last articles
Mg in 3C4JMg in 3C41
Mg in 3C3I
Mg in 3C1M
Mg in 3C3C
Mg in 3C2T
Mg in 3C3A
Mg in 3C15
Mg in 3C16
Mg in 3C14