Magnesium »
PDB 2oh7-2oup »
2ou7 »
Magnesium in PDB 2ou7: Structure of the Catalytic Domain of Human Polo-Like Kinase 1
Enzymatic activity of Structure of the Catalytic Domain of Human Polo-Like Kinase 1
All present enzymatic activity of Structure of the Catalytic Domain of Human Polo-Like Kinase 1:
2.7.11.21;
2.7.11.21;
Protein crystallography data
The structure of Structure of the Catalytic Domain of Human Polo-Like Kinase 1, PDB code: 2ou7
was solved by
Y.-H.Ding,
M.Kothe,
D.Kohls,
S.Low,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.90 / 2.40 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 65.958, 65.958, 154.045, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.3 / 23.5 |
Other elements in 2ou7:
The structure of Structure of the Catalytic Domain of Human Polo-Like Kinase 1 also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the Catalytic Domain of Human Polo-Like Kinase 1
(pdb code 2ou7). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of the Catalytic Domain of Human Polo-Like Kinase 1, PDB code: 2ou7:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of the Catalytic Domain of Human Polo-Like Kinase 1, PDB code: 2ou7:
Jump to Magnesium binding site number: 1; 2;
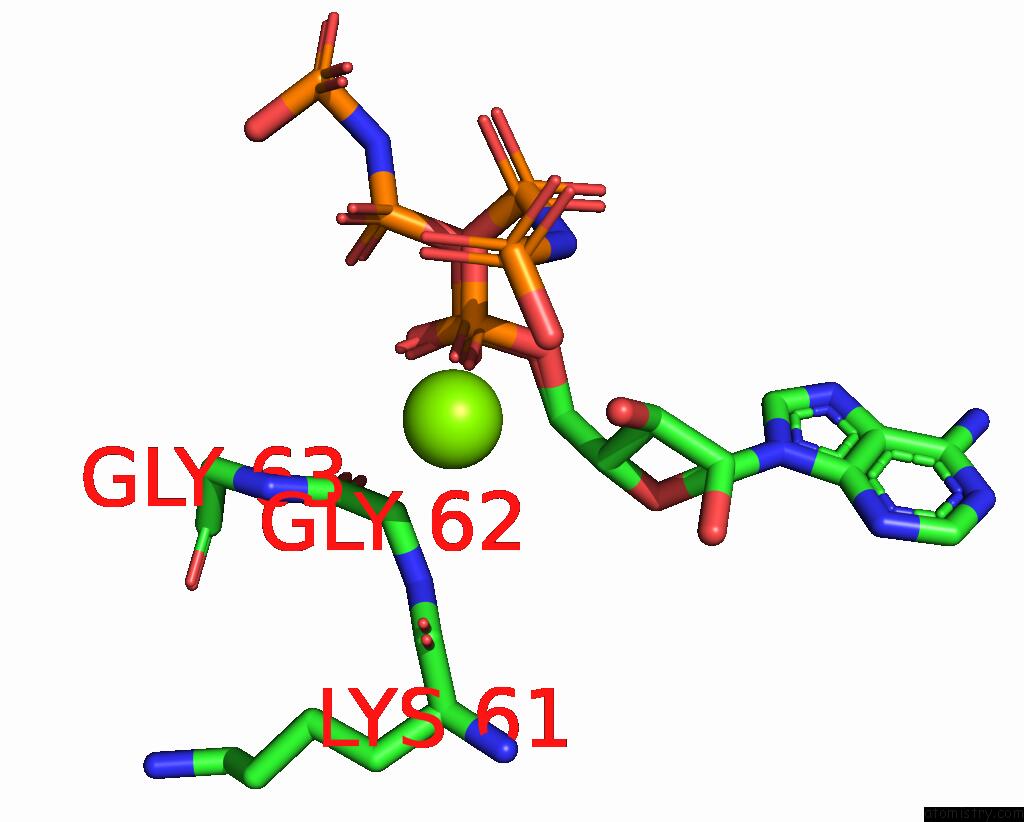
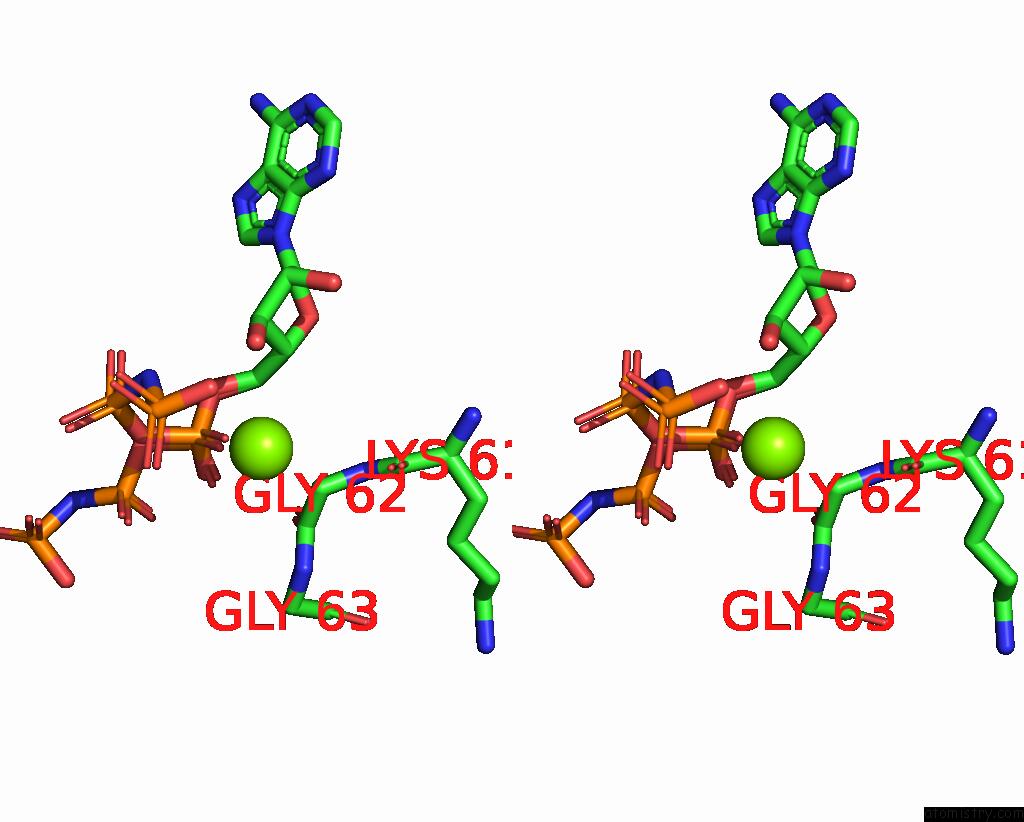
Magnesium binding site 1 out of 2 in 2ou7
Go back to
Magnesium binding site 1 out
of 2 in the Structure of the Catalytic Domain of Human Polo-Like Kinase 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the Catalytic Domain of Human Polo-Like Kinase 1 within 5.0Å range:
|
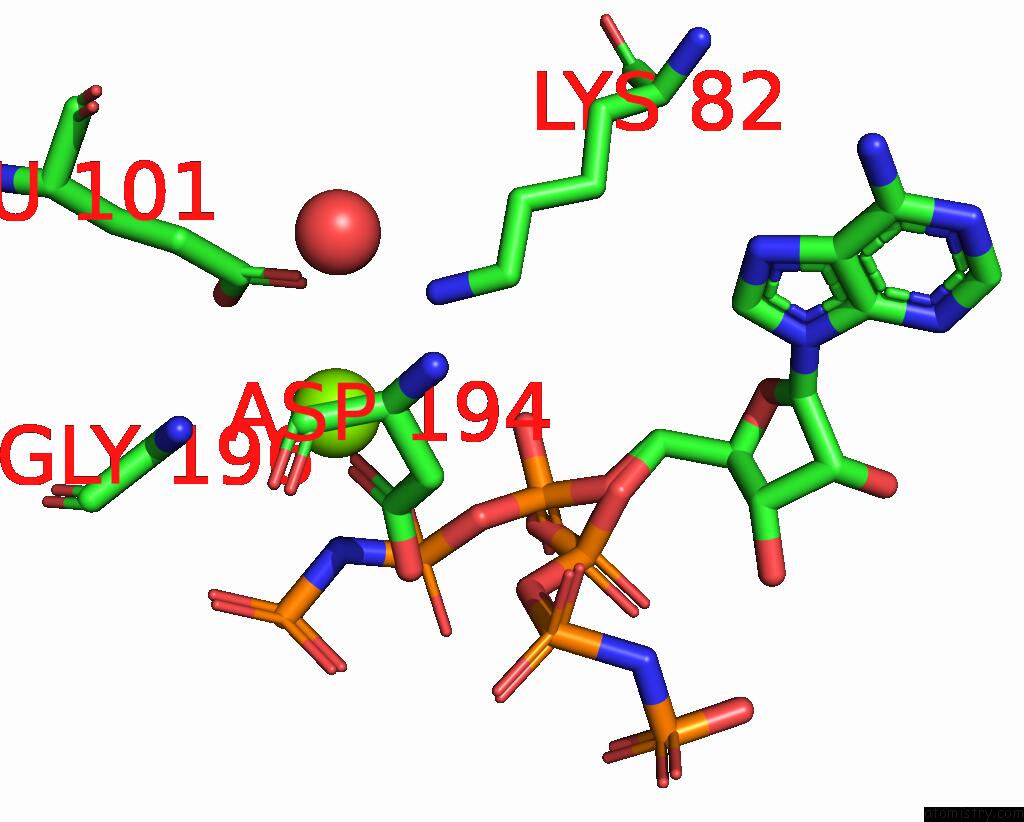
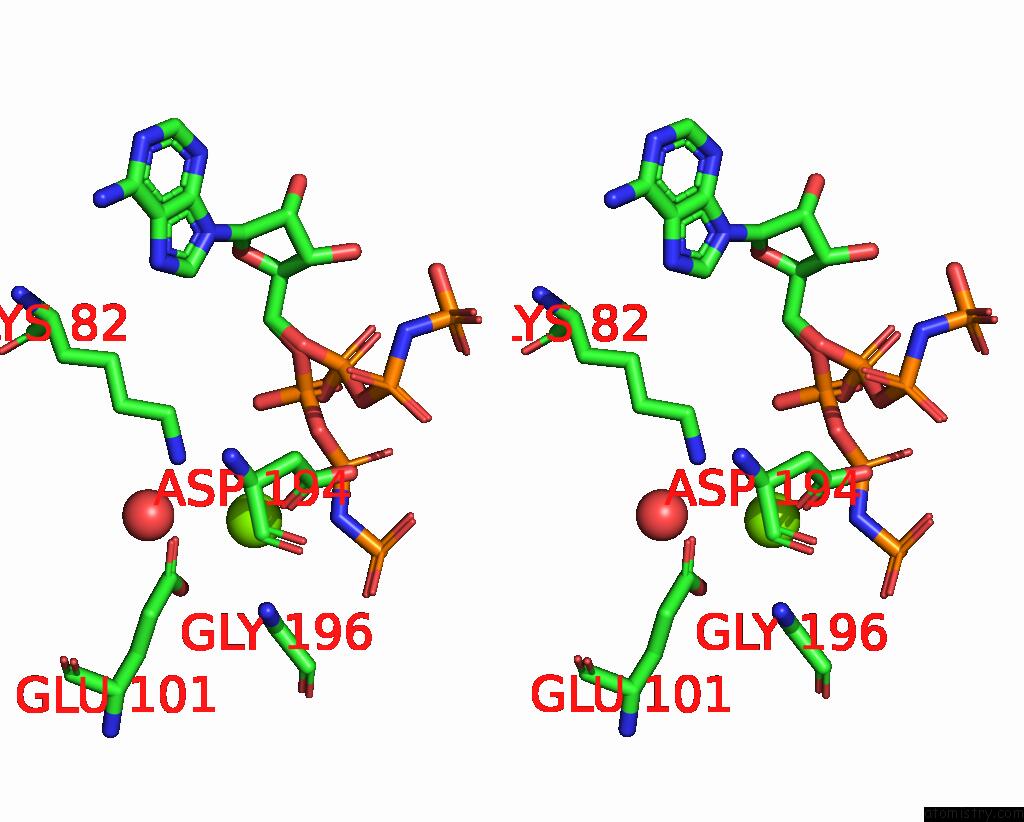
Magnesium binding site 2 out of 2 in 2ou7
Go back to
Magnesium binding site 2 out
of 2 in the Structure of the Catalytic Domain of Human Polo-Like Kinase 1
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of the Catalytic Domain of Human Polo-Like Kinase 1 within 5.0Å range:
|
Reference:
M.Kothe,
D.Kohls,
S.Low,
R.Coli,
A.C.Cheng,
S.L.Jacques,
T.L.Johnson,
C.Lewis,
C.Loh,
J.Nonomiya,
A.L.Sheils,
K.A.Verdries,
T.A.Wynn,
C.Kuhn,
Y.H.Ding.
Structure of the Catalytic Domain of Human Polo-Like Kinase 1. Biochemistry V. 46 5960 2007.
ISSN: ISSN 0006-2960
PubMed: 17461553
DOI: 10.1021/BI602474J
Page generated: Wed Aug 14 01:35:04 2024
ISSN: ISSN 0006-2960
PubMed: 17461553
DOI: 10.1021/BI602474J
Last articles
F in 4I0HF in 4I0J
F in 4HY5
F in 4HY6
F in 4HXN
F in 4HT2
F in 4HU1
F in 4HVS
F in 4HW7
F in 4HUA