Magnesium »
PDB 2uxj-2v7y »
2v0j »
Magnesium in PDB 2v0j: Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu)
Protein crystallography data
The structure of Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu), PDB code: 2v0j
was solved by
I.Mochalkin,
S.Lightle,
J.F.Ohren,
N.Y.Chirgadze,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.0 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 108.720, 108.720, 326.754, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.7 / 22.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu)
(pdb code 2v0j). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu), PDB code: 2v0j:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu), PDB code: 2v0j:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 2v0j
Go back to
Magnesium binding site 1 out
of 2 in the Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu)
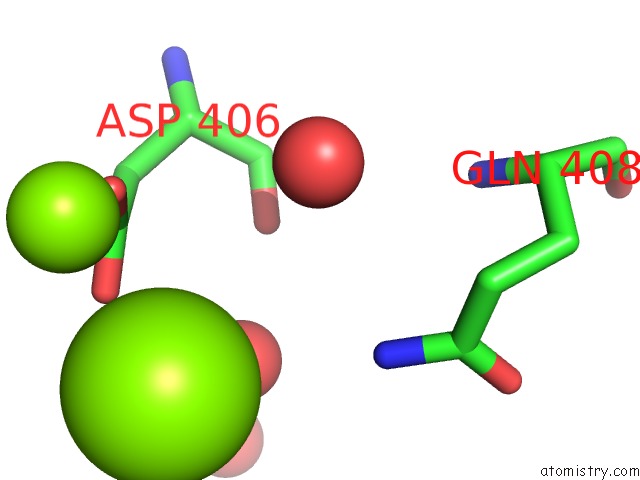
Mono view
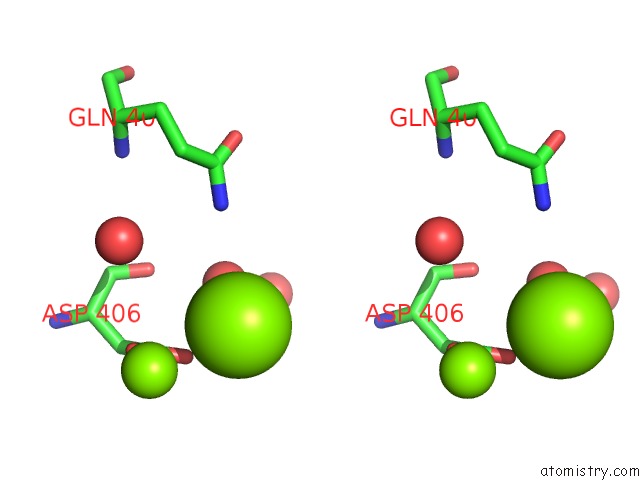
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu) within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 2v0j
Go back to
Magnesium binding site 2 out
of 2 in the Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu)
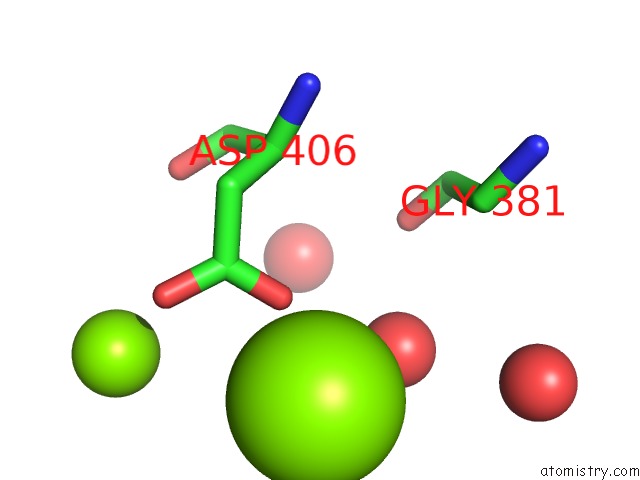
Mono view
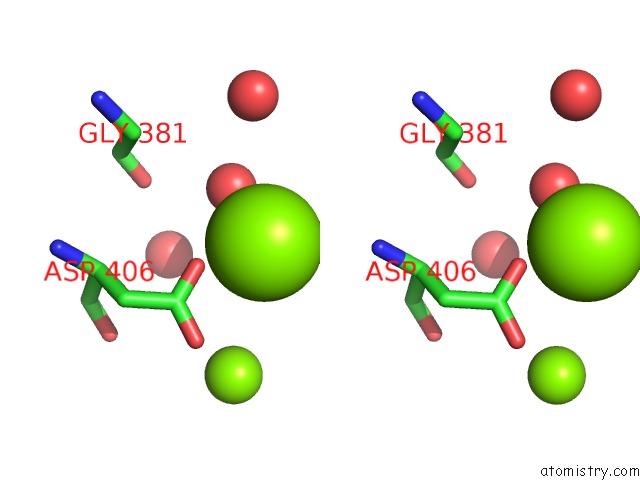
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Characterization of Substrate Binding and Catalysis of the Potential Antibacterial Target N-Acetylglucosamine-1- Phosphate Uridyltransferase (Glmu) within 5.0Å range:
|
Reference:
I.Mochalkin,
S.Lightle,
Y.Zhu,
J.F.Ohren,
C.Spessard,
N.Y.Chirgadze,
C.Banotai,
M.Melnick,
L.Mcdowell.
Characterization of Substrate Binding and Catalysis in the Potential Antibacterial Target N- Acetylglucosamine-1-Phosphate Uridyltransferase (Glmu). Protein Sci. V. 16 2657 2007.
ISSN: ISSN 0961-8368
PubMed: 18029420
DOI: 10.1110/PS.073135107
Page generated: Wed Aug 14 04:56:36 2024
ISSN: ISSN 0961-8368
PubMed: 18029420
DOI: 10.1110/PS.073135107
Last articles
Ca in 5SZLCa in 5SY1
Ca in 5SWI
Ca in 5SVE
Ca in 5SSX
Ca in 5SV0
Ca in 5STD
Ca in 5SSZ
Ca in 5SSY
Ca in 5SIC