Magnesium »
PDB 2vfx-2vsc »
2vpo »
Magnesium in PDB 2vpo: High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine
Protein crystallography data
The structure of High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine, PDB code: 2vpo
was solved by
S.I.Kuhlmann,
A.C.Terwisscha Van Scheltinga,
R.Bienert,
H.J.Kunte,
C.Ziegler,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.82 / 1.80 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.600, 52.100, 63.700, 80.80, 85.70, 78.00 |
| R / Rfree (%) | 17.9 / 22.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine
(pdb code 2vpo). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine, PDB code: 2vpo:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine, PDB code: 2vpo:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 2vpo
Go back to
Magnesium binding site 1 out
of 2 in the High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine
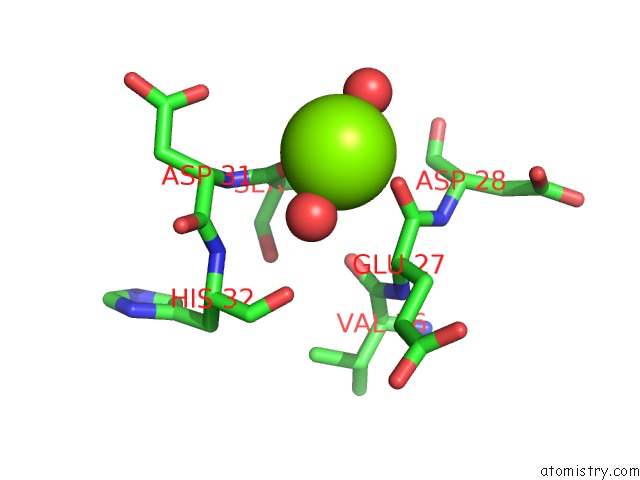
Mono view
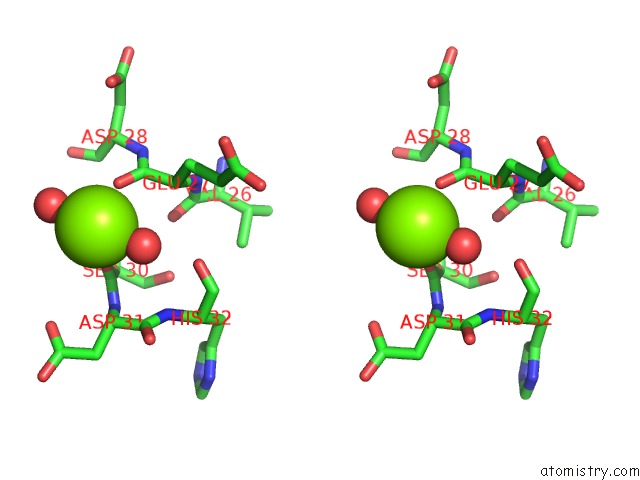
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 2vpo
Go back to
Magnesium binding site 2 out
of 2 in the High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine
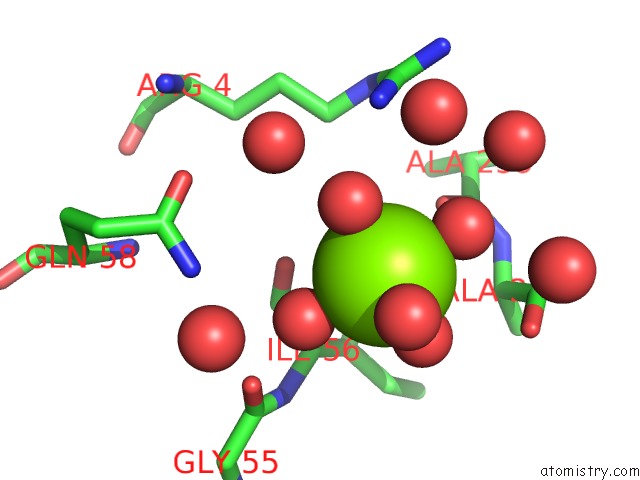
Mono view
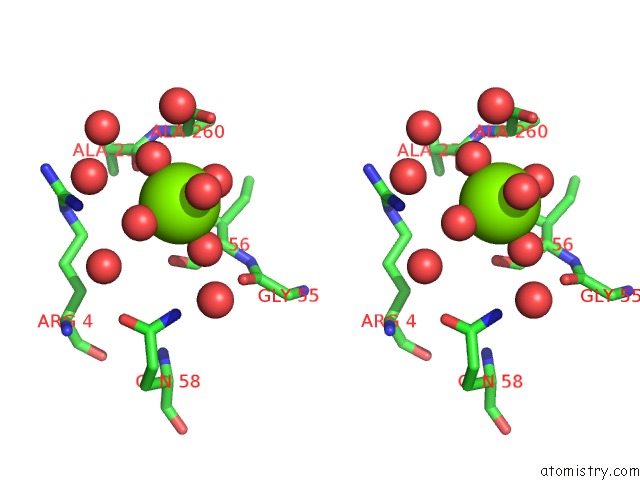
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of High Resolution Structure of the Periplasmic Binding Protein Teaa From Teaabc Trap Transporter of Halomonas Elongata in Complex with Hydroxyectoine within 5.0Å range:
|
Reference:
S.I.Kuhlmann,
A.C.Terwisscha Van Scheltinga,
R.Bienert,
H.J.Kunte,
C.Ziegler.
1.55 A Structure of the Ectoine Binding Protein Teaa of the Osmoregulated Trap-Transporter Teaabc From Halomonas Elongata. Biochemistry V. 47 9475 2008.
ISSN: ISSN 0006-2960
PubMed: 18702523
DOI: 10.1021/BI8006719
Page generated: Wed Aug 14 05:17:56 2024
ISSN: ISSN 0006-2960
PubMed: 18702523
DOI: 10.1021/BI8006719
Last articles
Ca in 5S60Ca in 5S61
Ca in 5S62
Ca in 5S5Z
Ca in 5S5X
Ca in 5S5Y
Ca in 5S5W
Ca in 5S5V
Ca in 5S5U
Ca in 5S5T