Magnesium »
PDB 2xni-2xzs »
2xul »
Magnesium in PDB 2xul: Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
Protein crystallography data
The structure of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations, PDB code: 2xul
was solved by
K.Zeth,
V.-R.Chellamuthu,
K.Forchhammer,
O.Fokina,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.09 / 2.20 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 71.870, 87.987, 116.341, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.929 / 22.488 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
(pdb code 2xul). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations, PDB code: 2xul:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations, PDB code: 2xul:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 2xul
Go back to
Magnesium binding site 1 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 2xul
Go back to
Magnesium binding site 2 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
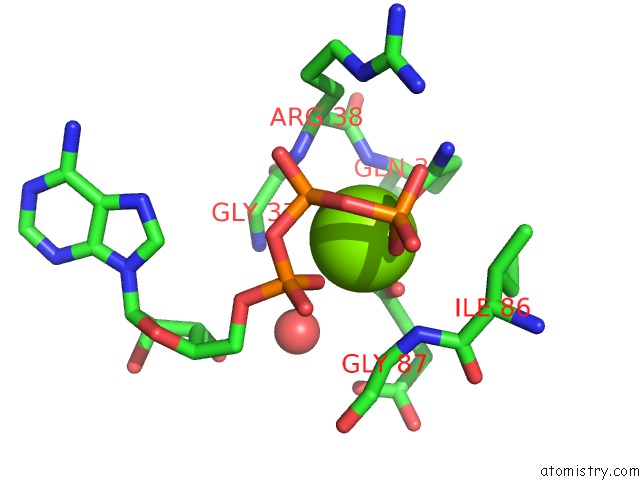
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 2xul
Go back to
Magnesium binding site 3 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
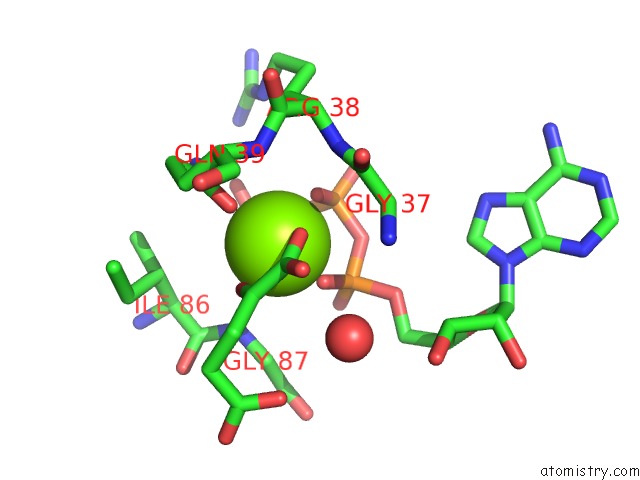
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 2xul
Go back to
Magnesium binding site 4 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
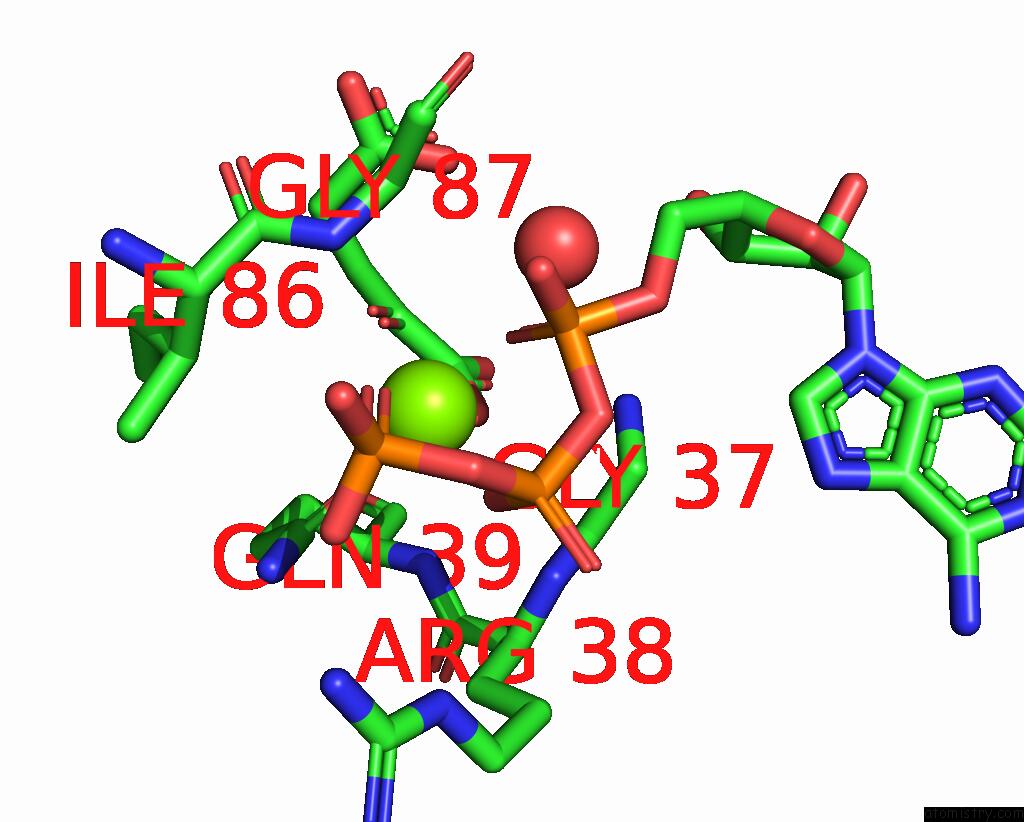
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 2xul
Go back to
Magnesium binding site 5 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations
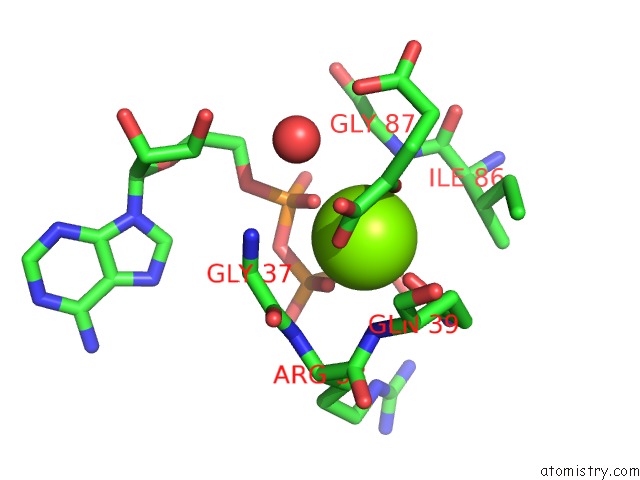
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 2xul
Go back to
Magnesium binding site 6 out
of 6 in the Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structure of Pii From Synechococcus Elongatus in Complex with 2-Oxoglutarate at High 2-Og Concentrations within 5.0Å range:
|
Reference:
O.Fokina,
V.-R.Chellamuthu,
K.Forchhammer,
K.Zeth.
Mechanism of 2-Oxoglutarate Signaling By the Synechococcus Elongatus Pii Signal Transduction Protein Proc.Natl.Acad.Sci.Usa V. 107 19760 2010.
ISSN: ISSN 0027-8424
PubMed: 21041661
DOI: 10.1073/PNAS.1007653107
Page generated: Wed Aug 14 07:18:43 2024
ISSN: ISSN 0027-8424
PubMed: 21041661
DOI: 10.1073/PNAS.1007653107
Last articles
Cl in 7SGVCl in 7SGY
Cl in 7SGX
Cl in 7SGW
Cl in 7SFO
Cl in 7SGU
Cl in 7SFL
Cl in 7SBC
Cl in 7SF6
Cl in 7SFI