Magnesium »
PDB 3a0u-3abk »
3a13 »
Magnesium in PDB 3a13: Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
Enzymatic activity of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
All present enzymatic activity of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca:
4.1.1.39;
4.1.1.39;
Protein crystallography data
The structure of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca, PDB code: 3a13
was solved by
Y.Nishitani,
M.Fujihashi,
T.Doi,
S.Yoshida,
H.Atomi,
T.Imanaka,
K.Miki,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.22 / 2.34 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 173.678, 247.090, 144.940, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.5 / 25 |
Other elements in 3a13:
The structure of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca also contains other interesting chemical elements:
| Calcium | (Ca) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
(pdb code 3a13). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 8 binding sites of Magnesium where determined in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca, PDB code: 3a13:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Magnesium where determined in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca, PDB code: 3a13:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Magnesium binding site 1 out of 8 in 3a13
Go back to
Magnesium binding site 1 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
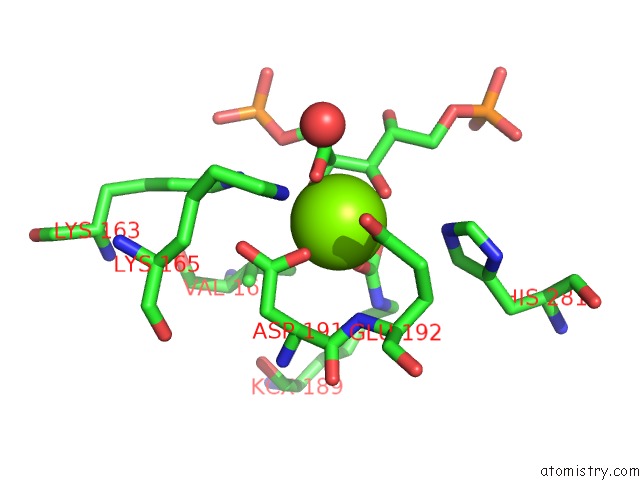
Mono view
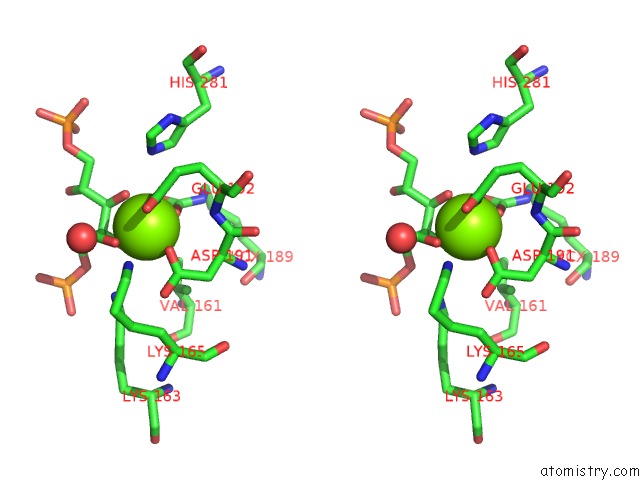
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 2 out of 8 in 3a13
Go back to
Magnesium binding site 2 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
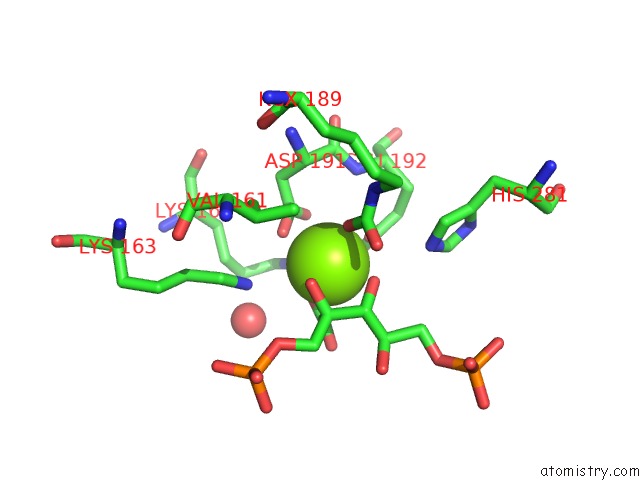
Mono view
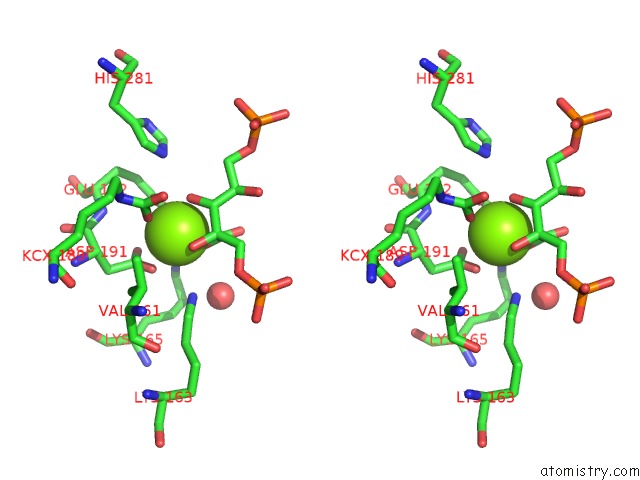
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 3 out of 8 in 3a13
Go back to
Magnesium binding site 3 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
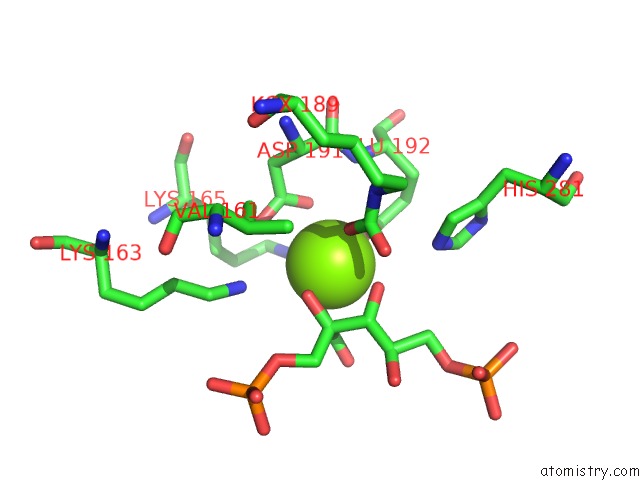
Mono view
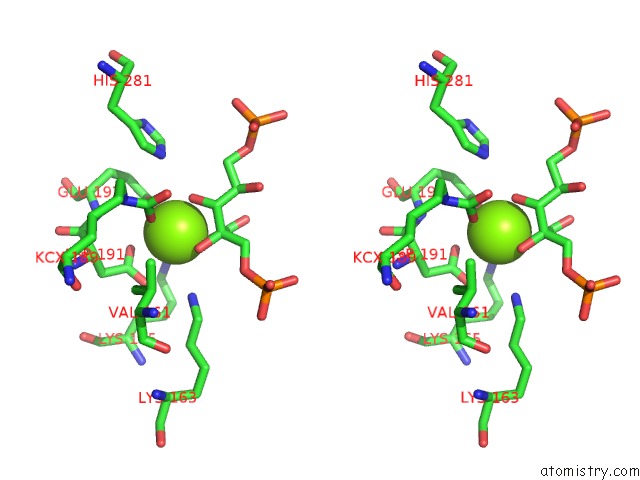
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 4 out of 8 in 3a13
Go back to
Magnesium binding site 4 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
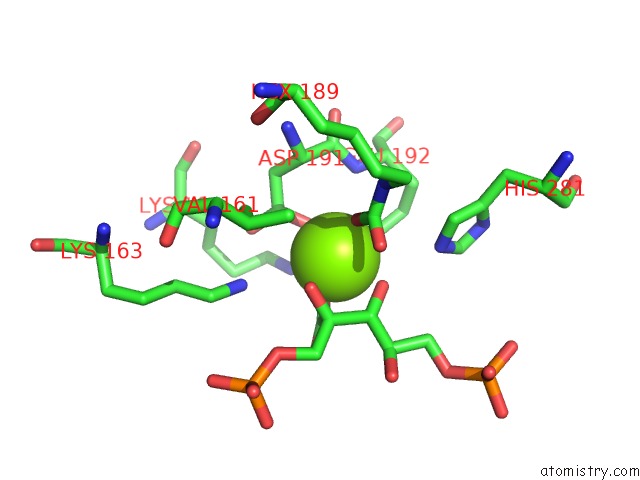
Mono view
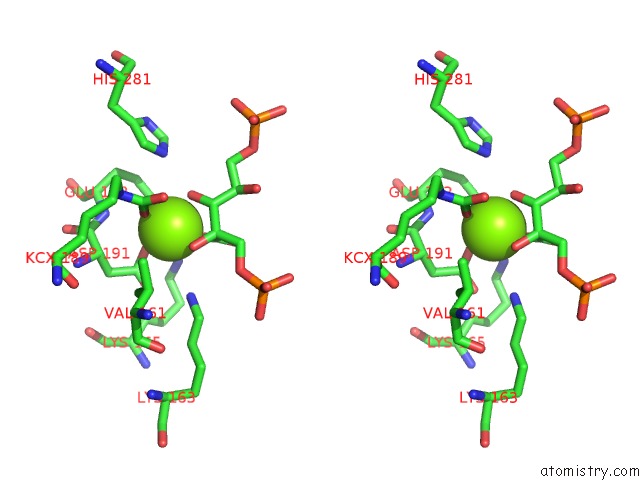
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 5 out of 8 in 3a13
Go back to
Magnesium binding site 5 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
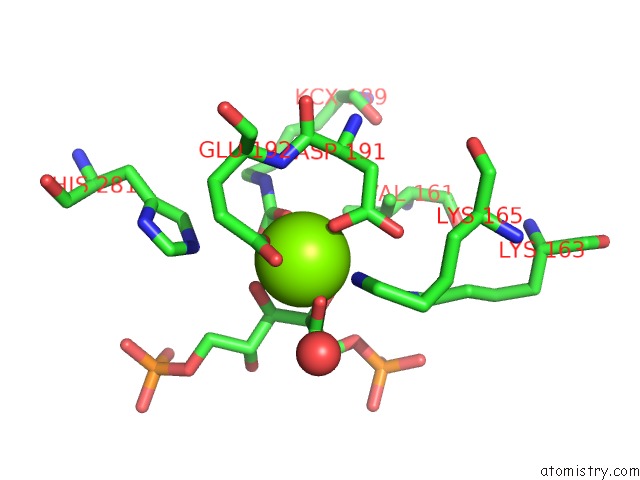
Mono view
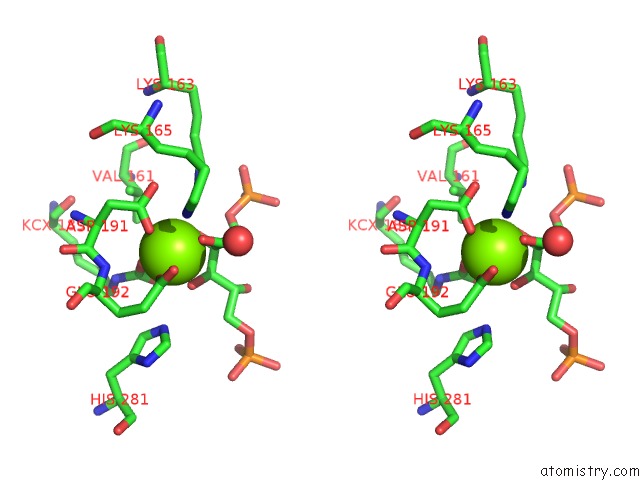
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 6 out of 8 in 3a13
Go back to
Magnesium binding site 6 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
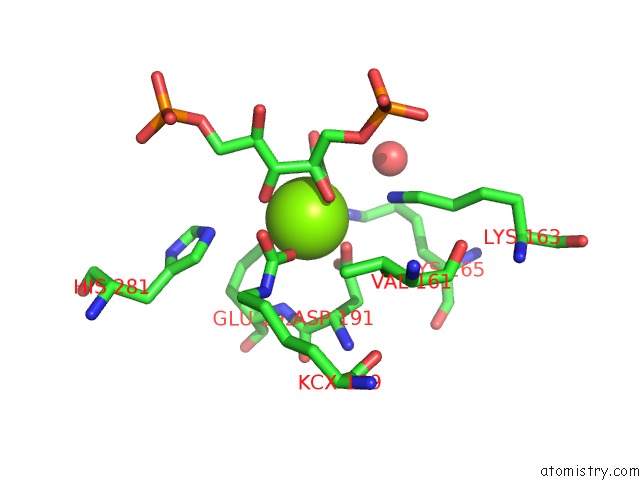
Mono view
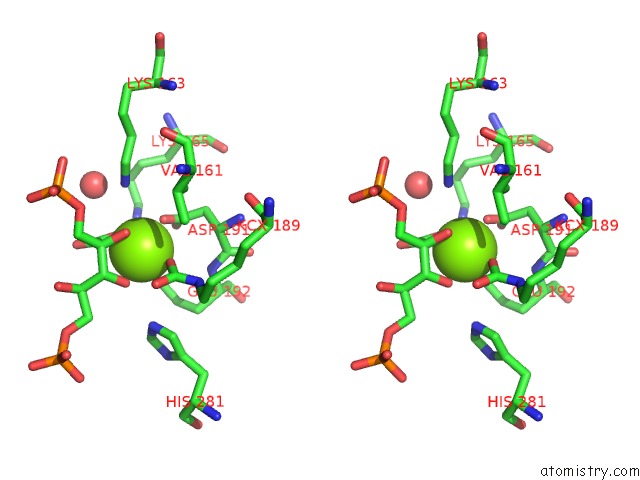
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 7 out of 8 in 3a13
Go back to
Magnesium binding site 7 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
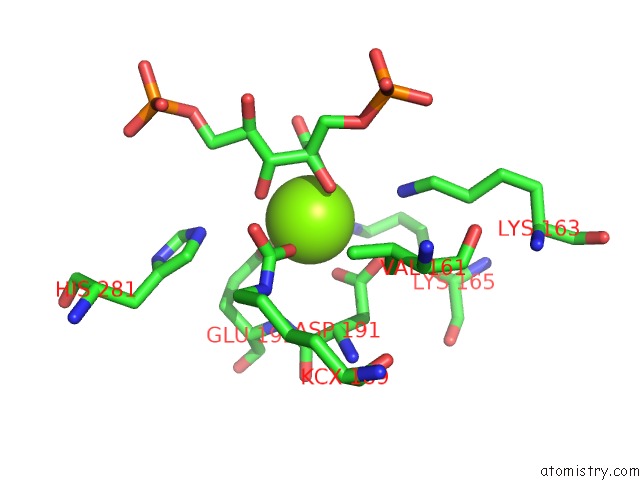
Mono view
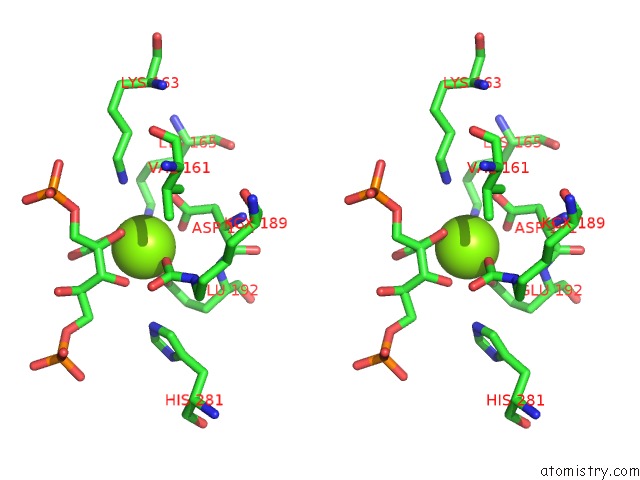
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Magnesium binding site 8 out of 8 in 3a13
Go back to
Magnesium binding site 8 out
of 8 in the Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca
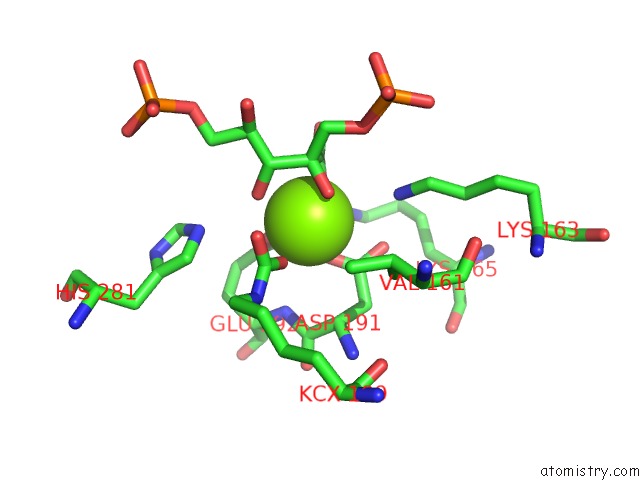
Mono view
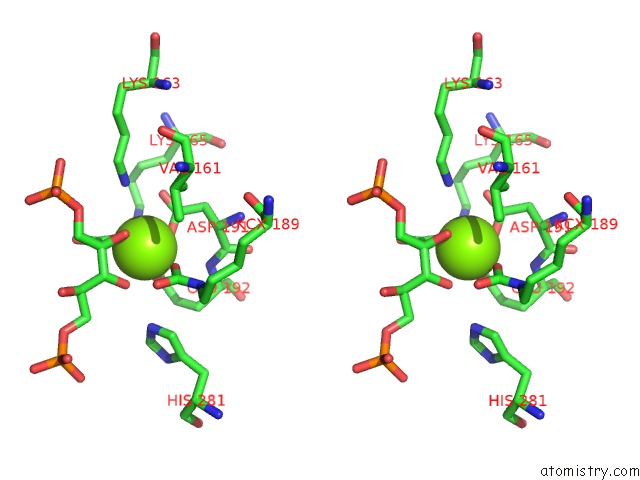
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Crystal Structure of Type III Rubisco SP4 Mutant Complexed with 2-Cabp and Activated with Ca within 5.0Å range:
|
Reference:
Y.Nishitani,
M.Fujihashi,
T.Doi,
S.Yoshida,
H.Atomi,
T.Imanaka,
K.Miki.
Sturcture-Based Optimization of A Type III Rubisco From A Hyperthermophile To Be Published.
Page generated: Sun Aug 10 17:19:54 2025
Last articles
Mg in 3JATMg in 3JAW
Mg in 3JAS
Mg in 3JAR
Mg in 3J81
Mg in 3JAO
Mg in 3JAL
Mg in 3JAK
Mg in 3JAA
Mg in 3J8I