Magnesium »
PDB 3a0u-3abk »
3a7d »
Magnesium in PDB 3a7d: Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor
Enzymatic activity of Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor
All present enzymatic activity of Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor:
2.1.1.6;
2.1.1.6;
Protein crystallography data
The structure of Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor, PDB code: 3a7d
was solved by
E.Tsuji,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 2.40 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.075, 58.082, 79.198, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.9 / 22.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor
(pdb code 3a7d). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor, PDB code: 3a7d:
In total only one binding site of Magnesium was determined in the Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor, PDB code: 3a7d:
Magnesium binding site 1 out of 1 in 3a7d
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor
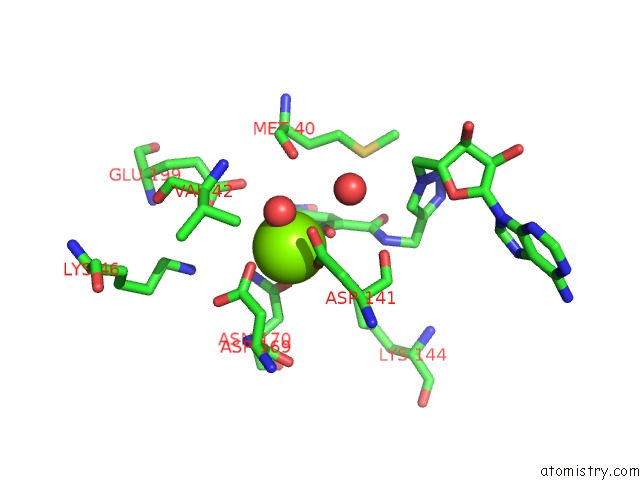
Mono view
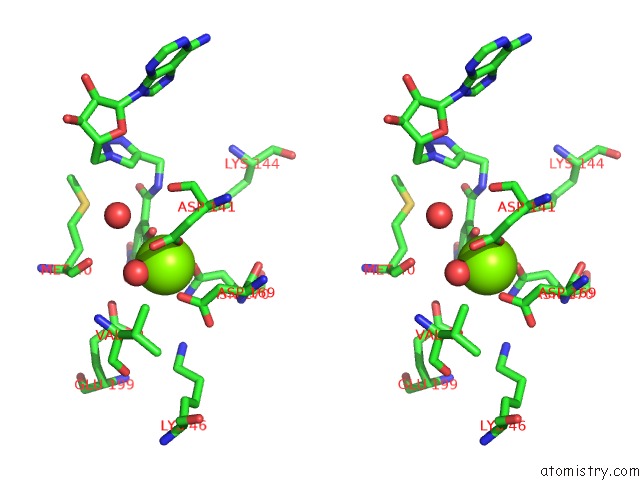
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structures of Rat Catechol-O-Methyltransferase Complexed with New Bi-Substrate Type Inhibitor within 5.0Å range:
|
Reference:
H.Muranaka,
M.Nakatsu,
E.Tsuji,
K.Okazaki.
Hit to Lead: Comprehensive Strategy of De Novo Scaffold Generation By Fbdd. Part 2: Ligand Fishing Using Mass Spectrometry By Detection of Ligand-Protein Non-Covalent Complex After Matrix Click Chemistry To Be Published.
Page generated: Sun Aug 10 17:23:22 2025
Last articles
Mg in 4E8NMg in 4E8P
Mg in 4E8M
Mg in 4E8G
Mg in 4E84
Mg in 4E89
Mg in 4DV7
Mg in 4E7Z
Mg in 4E6M
Mg in 4E7S