Magnesium »
PDB 3d1r-3dev »
3des »
Magnesium in PDB 3des: Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
Protein crystallography data
The structure of Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide, PDB code: 3des
was solved by
A.A.Fedorov,
E.V.Fedorov,
H.J.Imker,
J.A.Gerlt,
S.C.Almo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.92 / 2.30 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 191.672, 191.672, 283.085, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.3 / 23.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
(pdb code 3des). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide, PDB code: 3des:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide, PDB code: 3des:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 3des
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
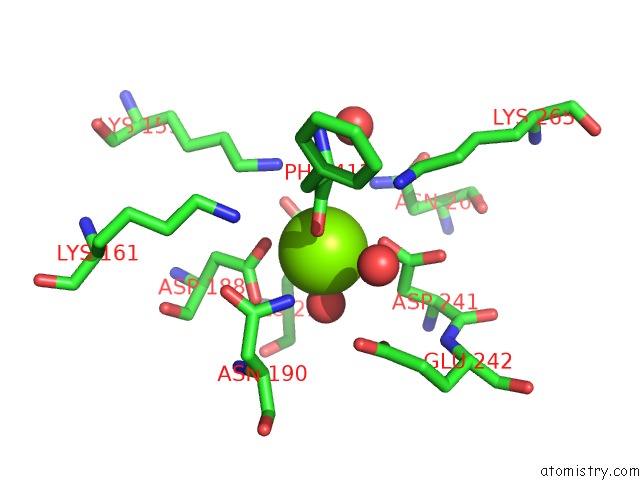
Mono view
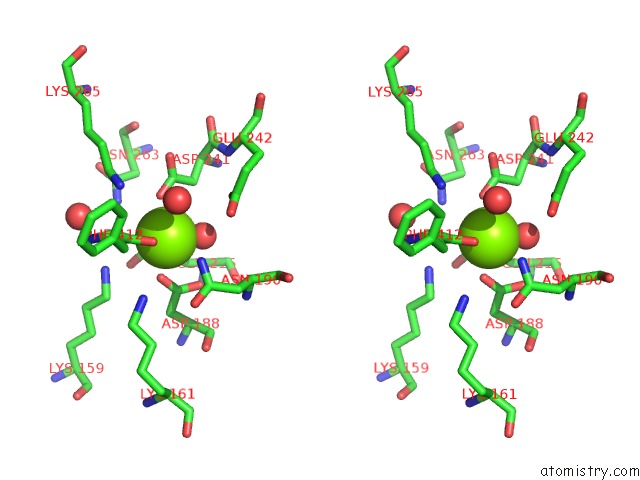
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 3des
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
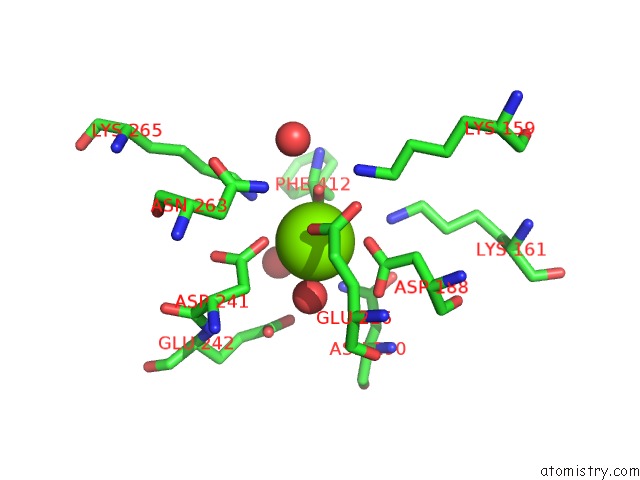
Mono view
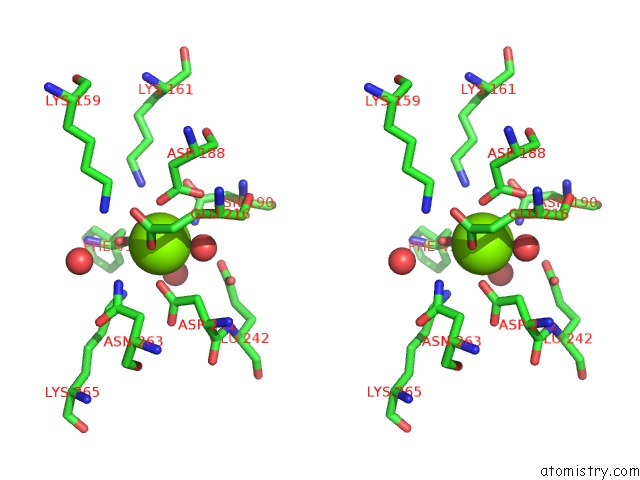
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 3des
Go back to
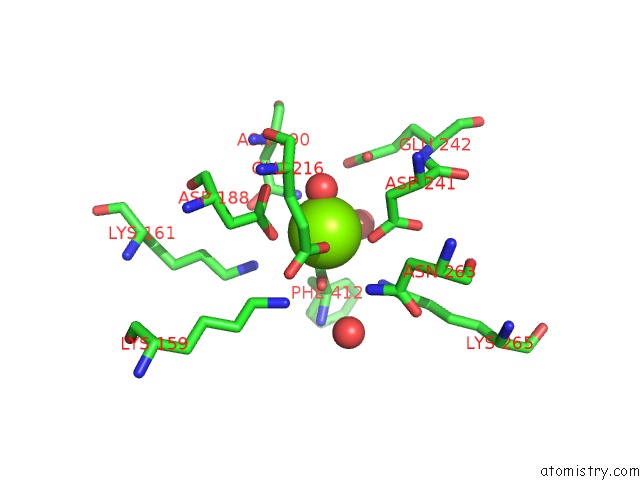
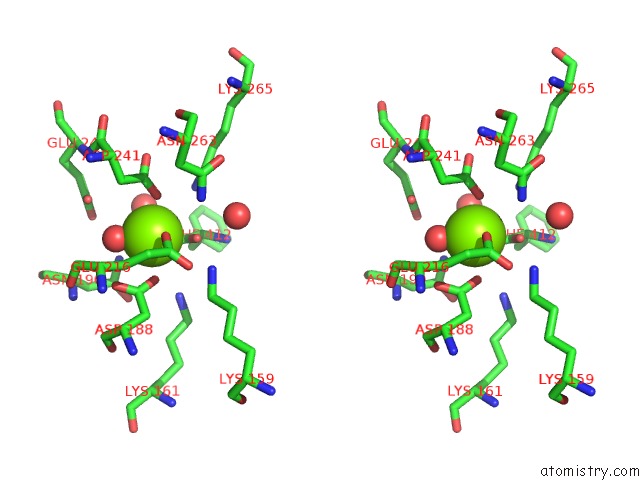
Magnesium binding site 3 out
of 4 in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 3des
Go back to
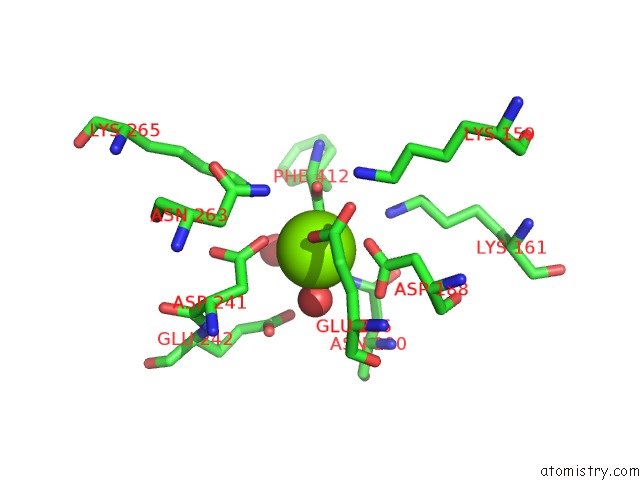
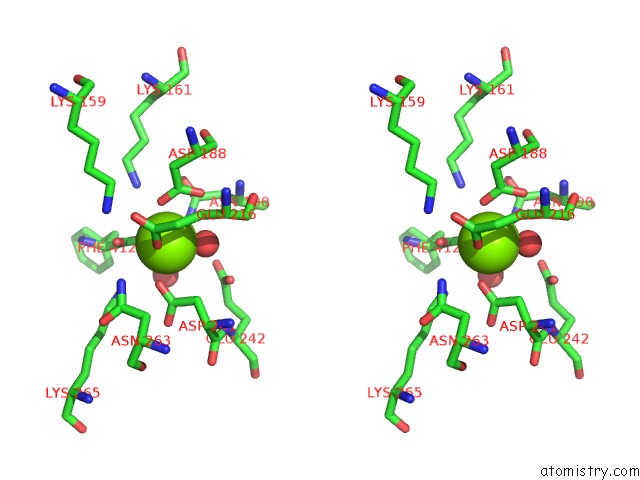
Magnesium binding site 4 out
of 4 in the Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Dipeptide Epimerase From Thermotoga Maritima Complexed with L-Ala-L-Phe Dipeptide within 5.0Å range:
|
Reference:
C.Kalyanaraman,
H.J.Imker,
A.A.Fedorov,
E.V.Fedorov,
M.E.Glasner,
P.C.Babbitt,
S.C.Almo,
J.A.Gerlt,
M.P.Jacobson.
Discovery of A Dipeptide Epimerase Enzymatic Function Guided By Homology Modeling and Virtual Screening. Structure V. 16 1668 2008.
ISSN: ISSN 0969-2126
PubMed: 19000819
DOI: 10.1016/J.STR.2008.08.015
Page generated: Wed Aug 14 12:28:36 2024
ISSN: ISSN 0969-2126
PubMed: 19000819
DOI: 10.1016/J.STR.2008.08.015
Last articles
F in 7L4CF in 7L24
F in 7L25
F in 7L1H
F in 7L0Z
F in 7L07
F in 7L01
F in 7L0E
F in 7L03
F in 7KYY