Magnesium »
PDB 3i5y-3ig8 »
3iaf »
Magnesium in PDB 3iaf: Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
Enzymatic activity of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
All present enzymatic activity of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate:
4.1.2.38;
4.1.2.38;
Protein crystallography data
The structure of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate, PDB code: 3iaf
was solved by
G.S.Brandt,
G.A.Petsko,
D.Ringe,
M.J.Mcleish,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.89 / 2.80 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 150.962, 150.962, 195.560, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.3 / 26 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
(pdb code 3iaf). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate, PDB code: 3iaf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate, PDB code: 3iaf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 3iaf
Go back to
Magnesium binding site 1 out
of 4 in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
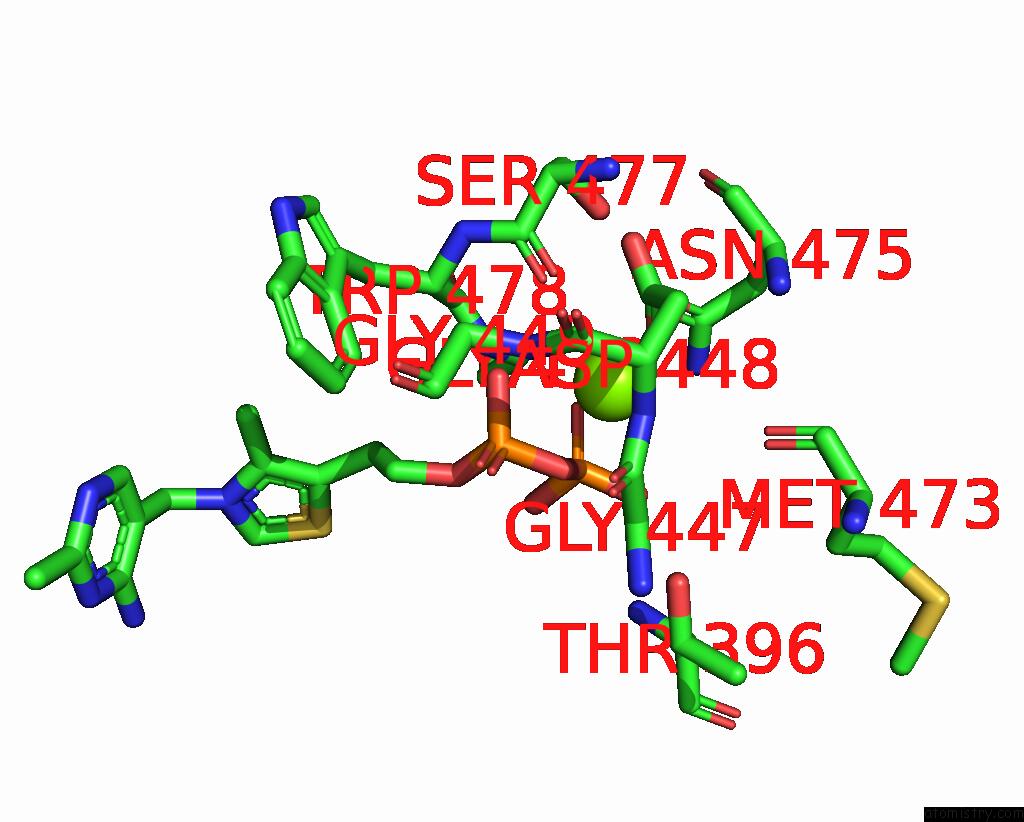
Mono view
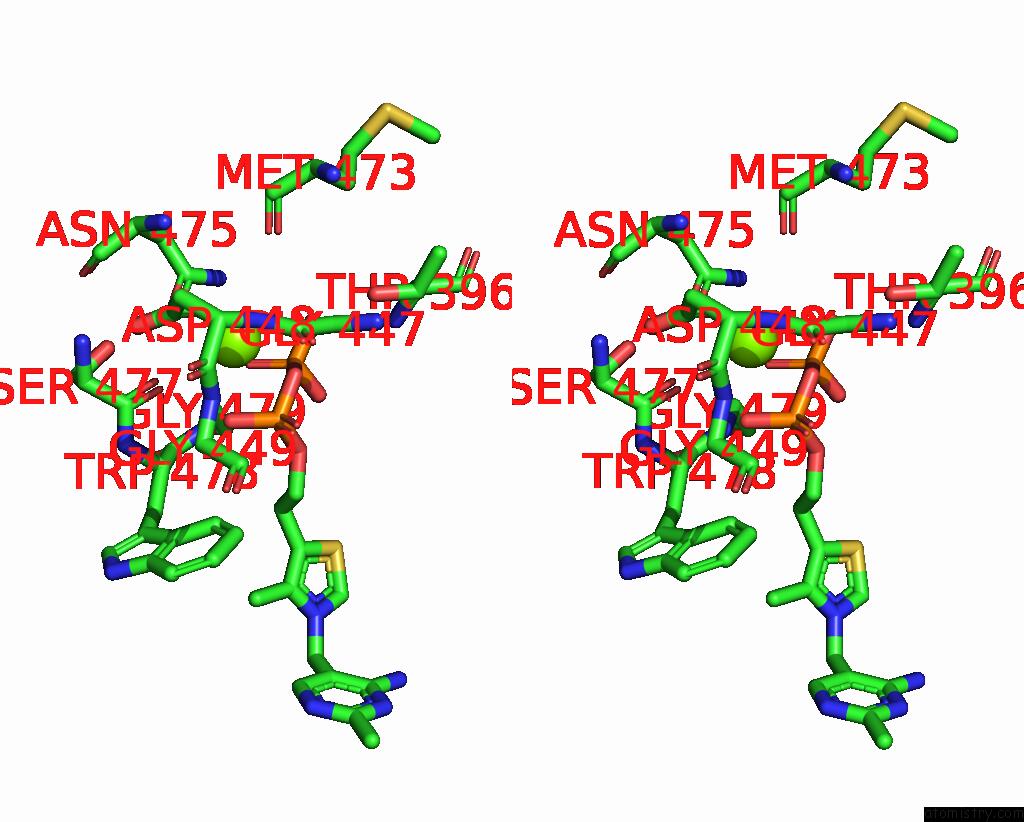
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 3iaf
Go back to
Magnesium binding site 2 out
of 4 in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
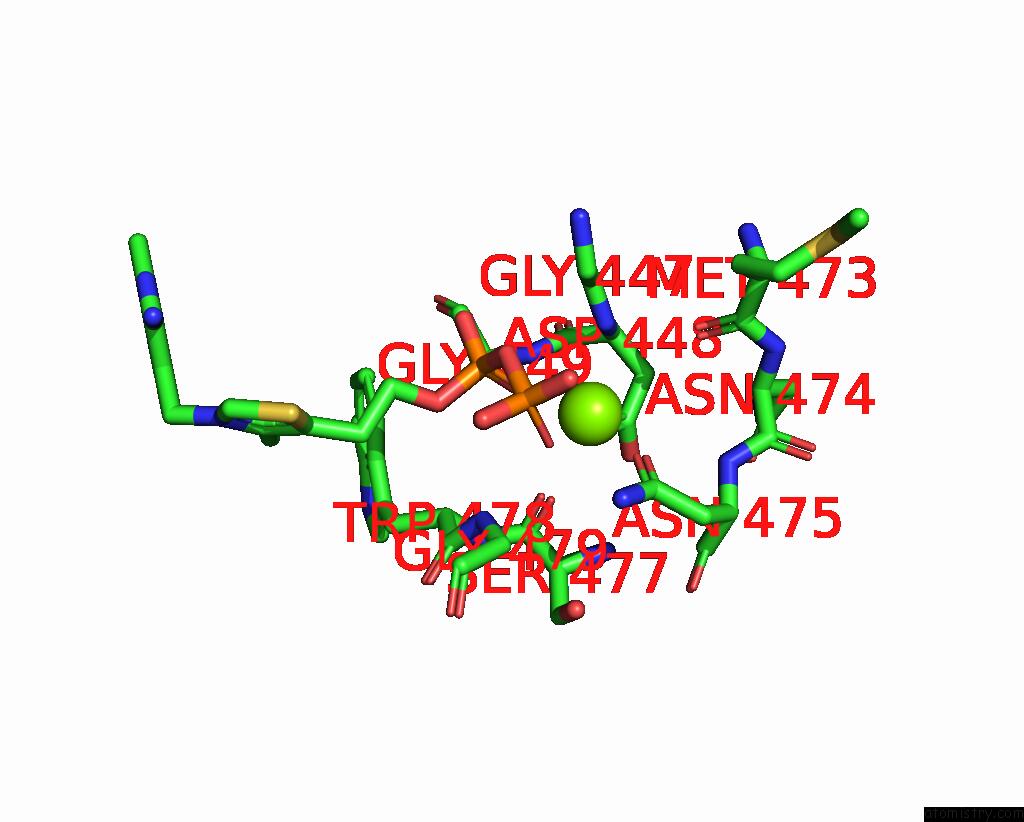
Mono view
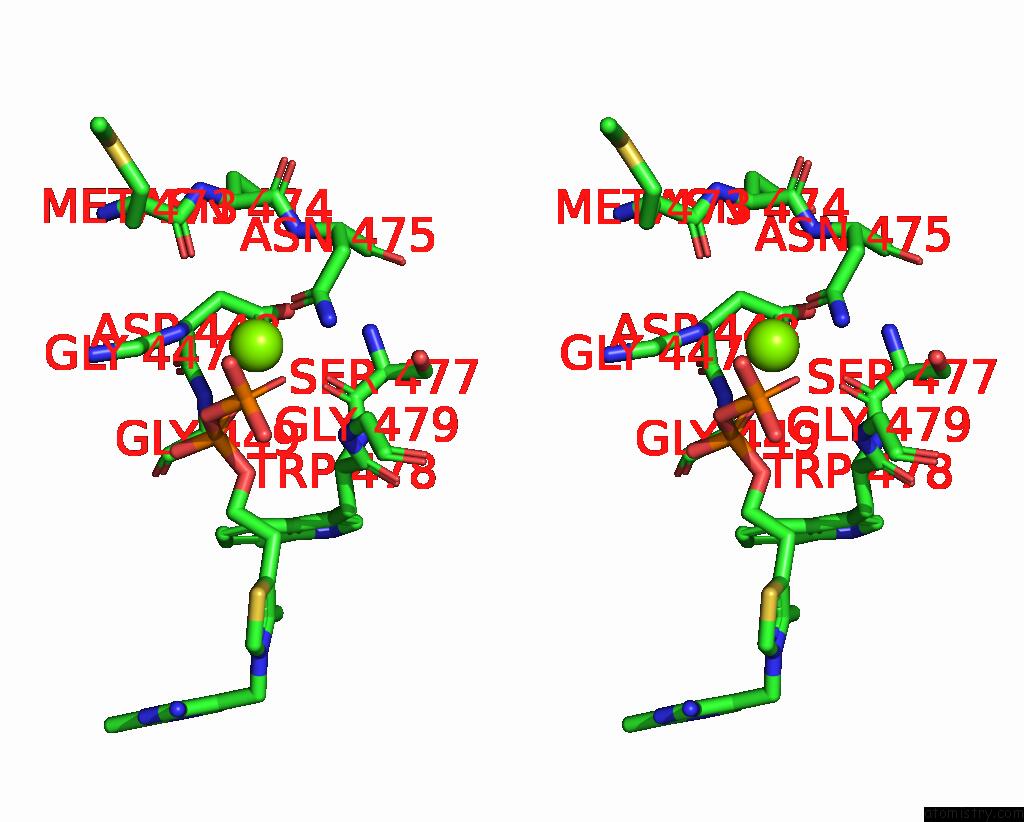
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate within 5.0Å range:
|
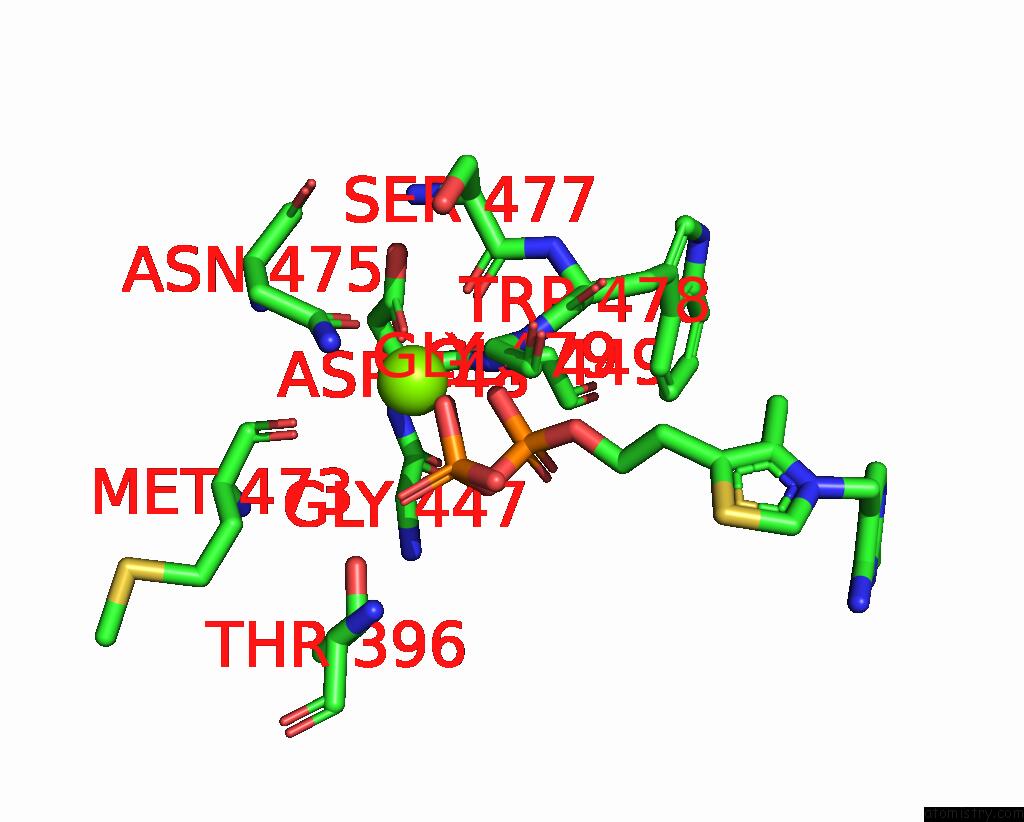
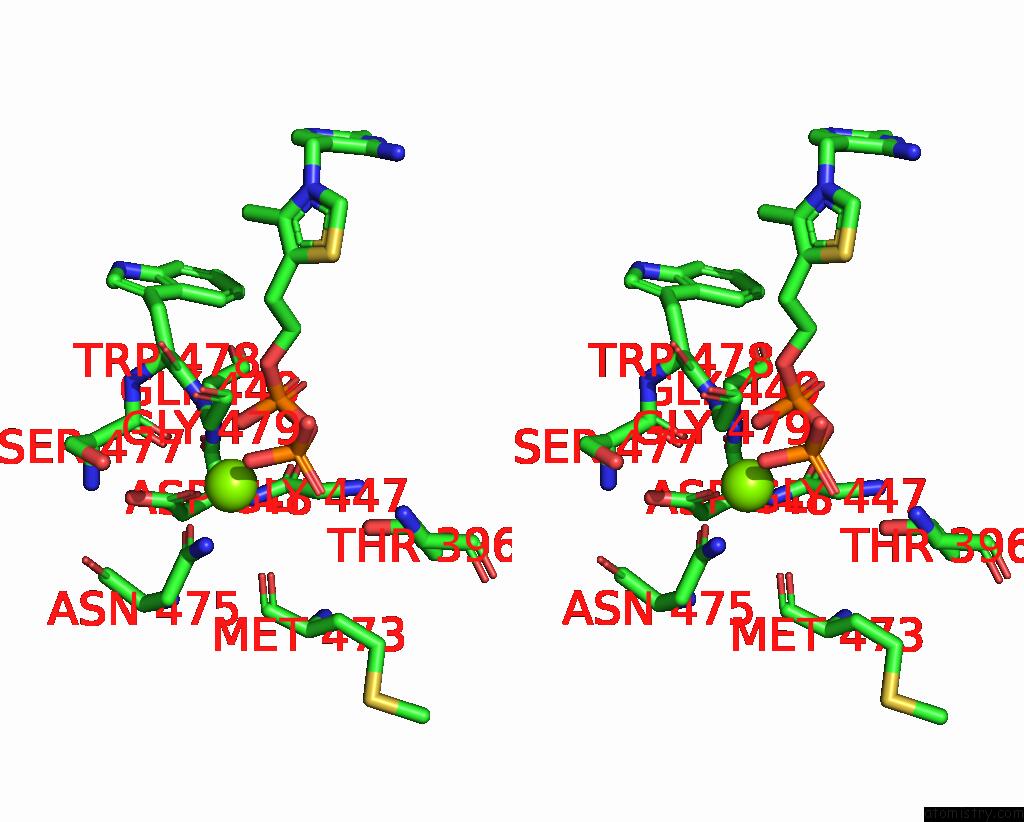
Magnesium binding site 3 out of 4 in 3iaf
Go back to
Magnesium binding site 3 out
of 4 in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate within 5.0Å range:
|
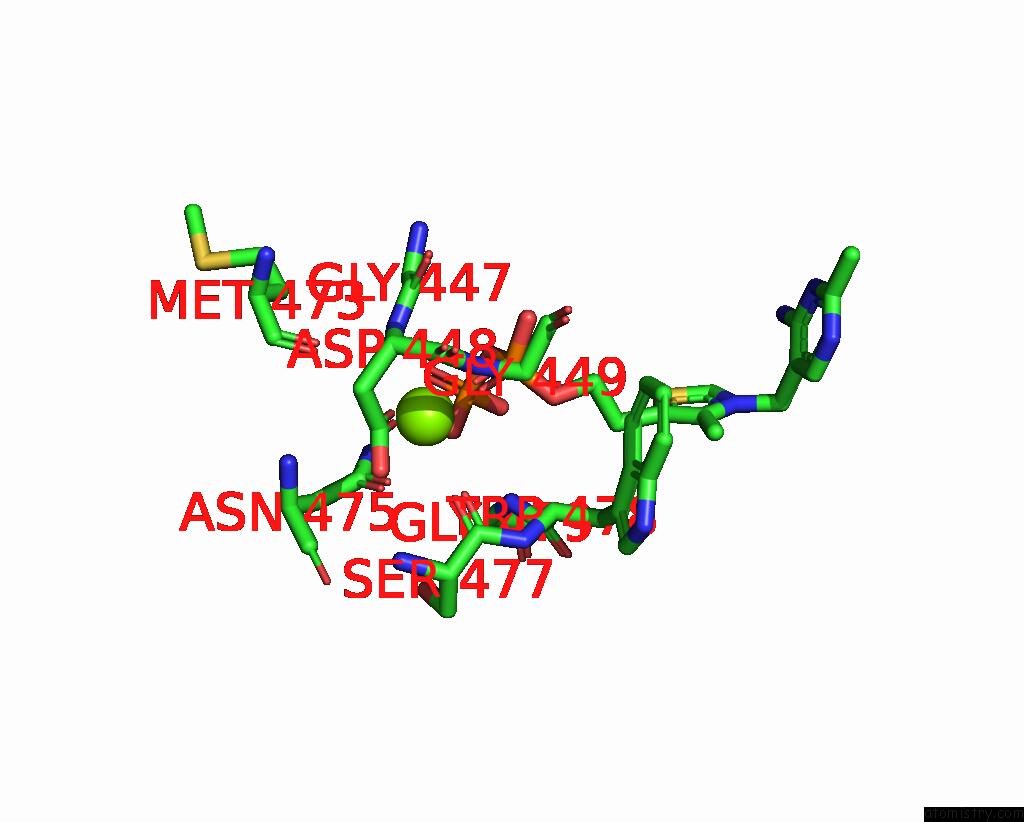
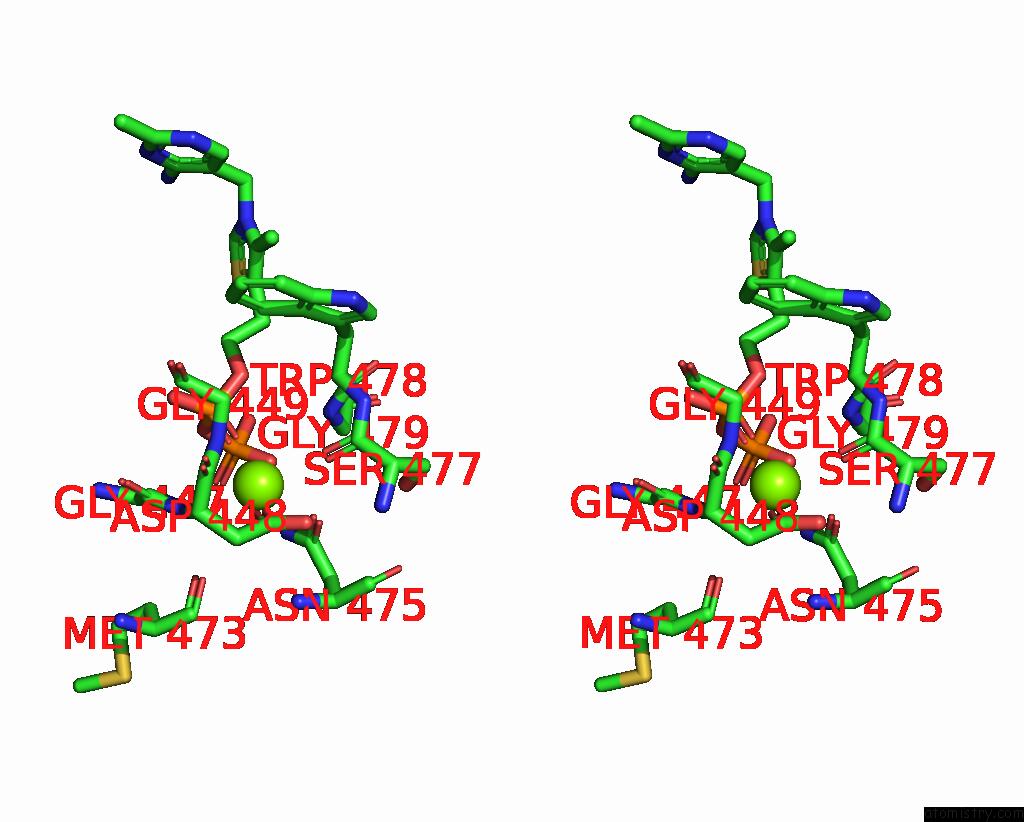
Magnesium binding site 4 out of 4 in 3iaf
Go back to
Magnesium binding site 4 out
of 4 in the Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Benzaldehyde Lyase A28S Mutant with Monomethyl Benzoylphosphonate within 5.0Å range:
|
Reference:
G.S.Brandt,
M.M.Kneen,
G.A.Petsko,
D.Ringe,
M.J.Mcleish.
Active-Site Engineering of Benzaldehyde Lyase Shows That A Point Mutation Can Confer Both New Reactivity and Susceptibility to Mechanism-Based Inhibition. J.Am.Chem.Soc. V. 132 438 2010.
ISSN: ISSN 0002-7863
PubMed: 20030408
DOI: 10.1021/JA907064W
Page generated: Sun Aug 10 22:30:16 2025
ISSN: ISSN 0002-7863
PubMed: 20030408
DOI: 10.1021/JA907064W
Last articles
Mg in 6LU1Mg in 6LT4
Mg in 6LY7
Mg in 6LY6
Mg in 6LY3
Mg in 6LX1
Mg in 6LTW
Mg in 6LVW
Mg in 6LUH
Mg in 6LTS