Magnesium »
PDB 3py8-3q86 »
3q43 »
Magnesium in PDB 3q43: X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
Protein crystallography data
The structure of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15, PDB code: 3q43
was solved by
S.Mcgowan,
D.C.Greenbaum,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.07 / 1.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.772, 108.814, 118.683, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.6 / 20.7 |
Other elements in 3q43:
The structure of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15 also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
(pdb code 3q43). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15, PDB code: 3q43:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15, PDB code: 3q43:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 3q43
Go back to
Magnesium binding site 1 out
of 4 in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
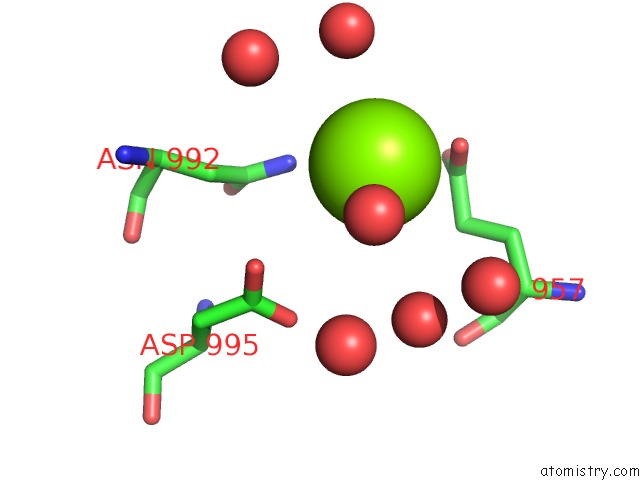
Mono view
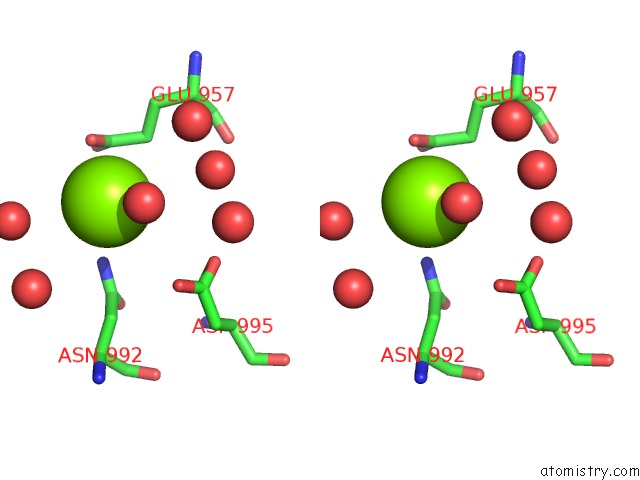
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15 within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 3q43
Go back to
Magnesium binding site 2 out
of 4 in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
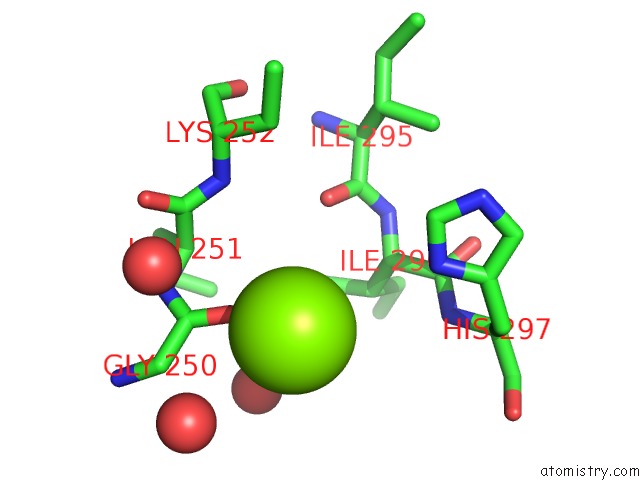
Mono view
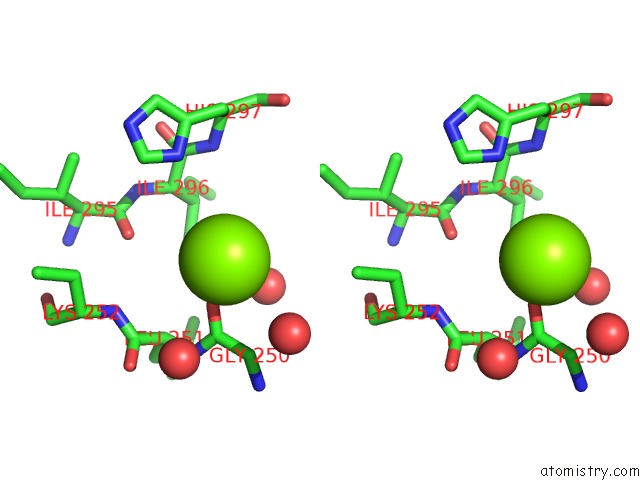
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15 within 5.0Å range:
|
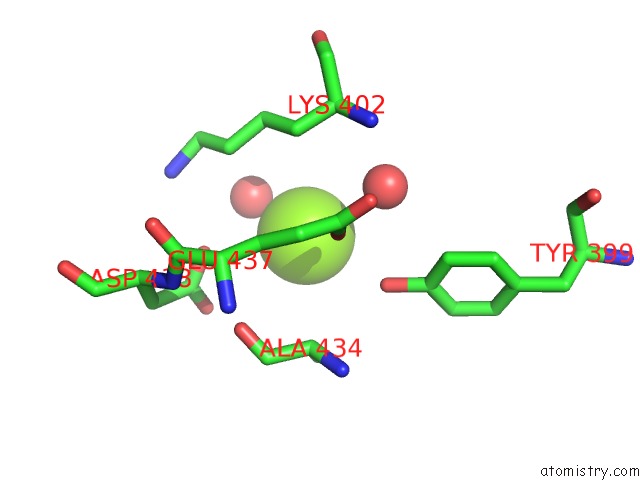
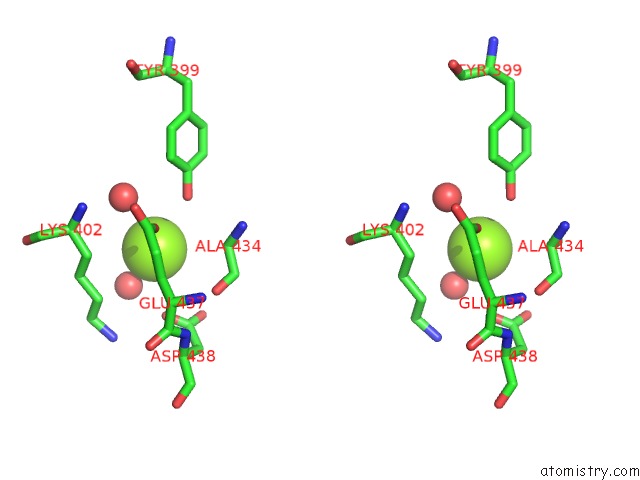
Magnesium binding site 3 out of 4 in 3q43
Go back to
Magnesium binding site 3 out
of 4 in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15 within 5.0Å range:
|
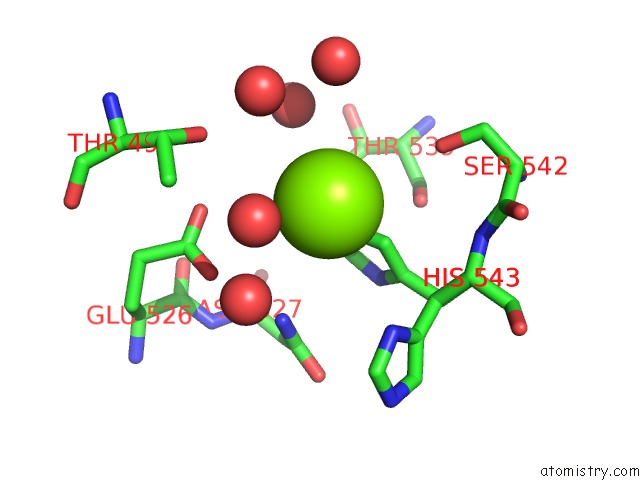
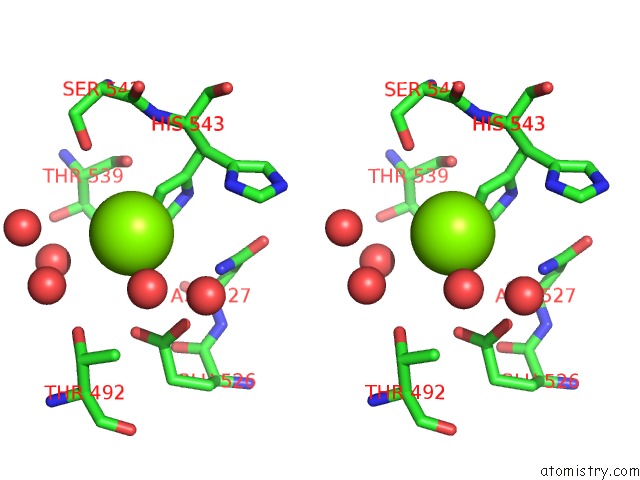
Magnesium binding site 4 out of 4 in 3q43
Go back to
Magnesium binding site 4 out
of 4 in the X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of X-Ray Crystal Structure of Pfa-M1 Bound to Bestatin Derivative 15 within 5.0Å range:
|
Reference:
G.Velmourougane,
M.B.Harbut,
S.Dalal,
S.Mcgowan,
C.A.Oellig,
N.Meinhardt,
J.C.Whisstock,
M.Klemba,
D.C.Greenbaum.
Synthesis of New (-)-Bestatin-Based Inhibitor Libraries Reveals A Novel Binding Mode in the S1 Pocket of the Essential Malaria M1 Metalloaminopeptidase. J.Med.Chem. V. 54 1655 2011.
ISSN: ISSN 0022-2623
PubMed: 21366301
DOI: 10.1021/JM101227T
Page generated: Thu Aug 15 09:53:58 2024
ISSN: ISSN 0022-2623
PubMed: 21366301
DOI: 10.1021/JM101227T
Last articles
F in 4LTSF in 4LUD
F in 4LUV
F in 4LOQ
F in 4LQG
F in 4LPB
F in 4LOP
F in 4LP0
F in 4LOY
F in 4LMN