Magnesium »
PDB 3zx5-4a2b »
3zyc »
Magnesium in PDB 3zyc: Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp
Protein crystallography data
The structure of Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp, PDB code: 3zyc
was solved by
J.S.Chappie,
J.A.Mears,
S.Fang,
M.Leonard,
S.L.Schmid,
R.A.Milligan,
J.E.Hinshaw,
F.Dyda,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.20 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 44.151, 82.635, 95.014, 90.00, 96.68, 90.00 |
| R / Rfree (%) | 22.5 / 26.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp
(pdb code 3zyc). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp, PDB code: 3zyc:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp, PDB code: 3zyc:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 3zyc
Go back to
Magnesium binding site 1 out
of 2 in the Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp
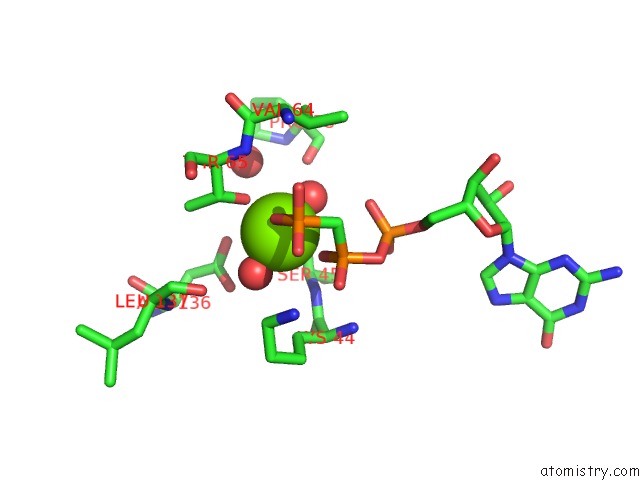
Mono view
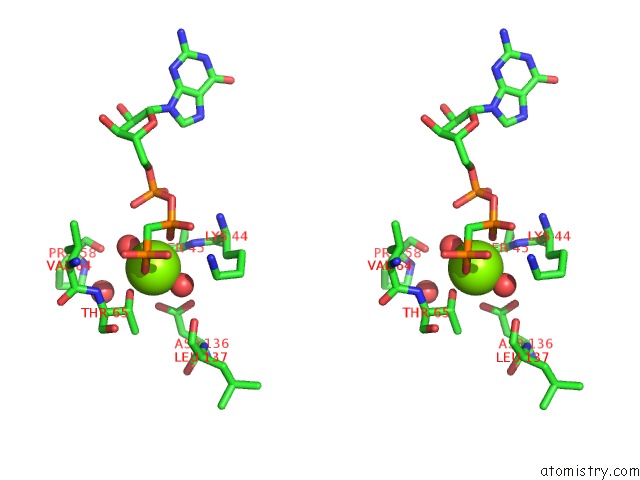
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 3zyc
Go back to
Magnesium binding site 2 out
of 2 in the Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp
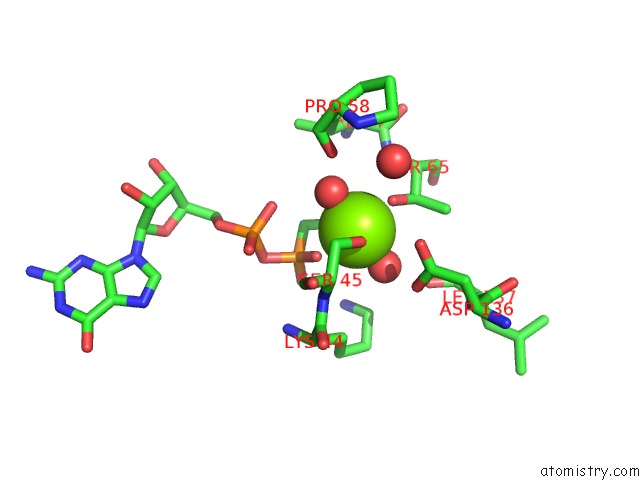
Mono view
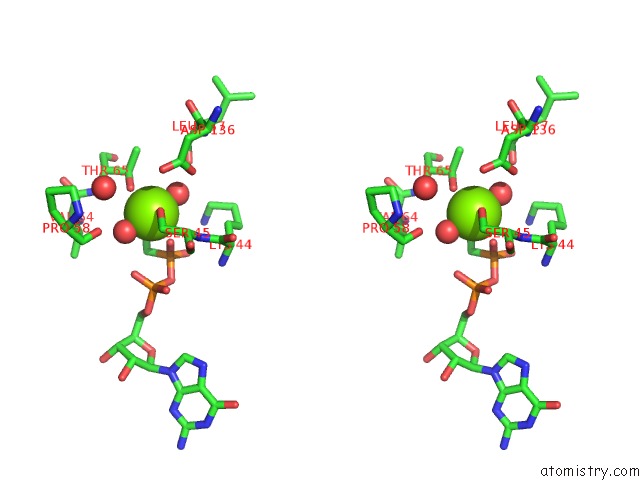
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Dynamin 1 Gtpase Ged Fusion Dimer Complexed with Gmppcp within 5.0Å range:
|
Reference:
J.S.Chappie,
J.A.Mears,
S.Fang,
M.Leonard,
S.L.Schmid,
R.A.Milligan,
J.E.Hinshaw,
F.Dyda.
A Pseudoatomic Model of the Dynamin Polymer Identifies A Hydrolysis-Dependent Powerstroke. Cell(Cambridge,Mass.) V. 147 209 2011.
ISSN: ISSN 0092-8674
PubMed: 21962517
DOI: 10.1016/J.CELL.2011.09.003
Page generated: Mon Aug 11 05:25:54 2025
ISSN: ISSN 0092-8674
PubMed: 21962517
DOI: 10.1016/J.CELL.2011.09.003
Last articles
Mg in 4DPGMg in 4DQP
Mg in 4DQQ
Mg in 4DPM
Mg in 4DPV
Mg in 4DQI
Mg in 4DOB
Mg in 4DOC
Mg in 4DMZ
Mg in 4DOA