Magnesium »
PDB 3zx5-4a2b »
4a0g »
Magnesium in PDB 4a0g: Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form.
Protein crystallography data
The structure of Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form., PDB code: 4a0g
was solved by
D.Cobessi,
R.Dumas,
V.Pautre,
C.Meinguet,
J.L.Ferrer,
C.Alban,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.574 / 2.50 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.442, 80.066, 136.939, 99.96, 107.12, 97.25 |
| R / Rfree (%) | 17.72 / 23.91 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form.
(pdb code 4a0g). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form., PDB code: 4a0g:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form., PDB code: 4a0g:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4a0g
Go back to
Magnesium binding site 1 out
of 2 in the Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form.
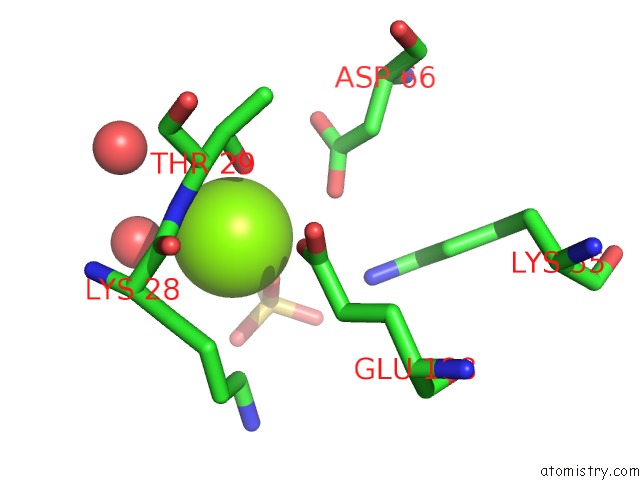
Mono view
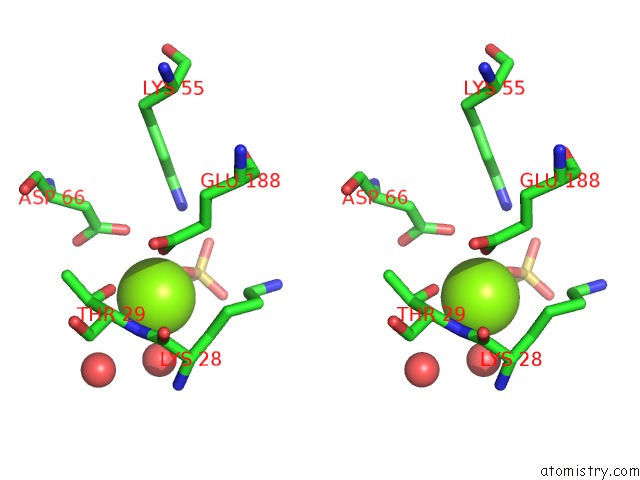
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form. within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4a0g
Go back to
Magnesium binding site 2 out
of 2 in the Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form.
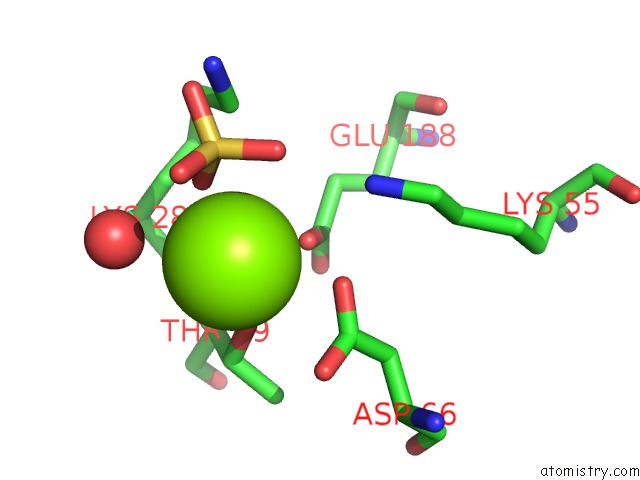
Mono view
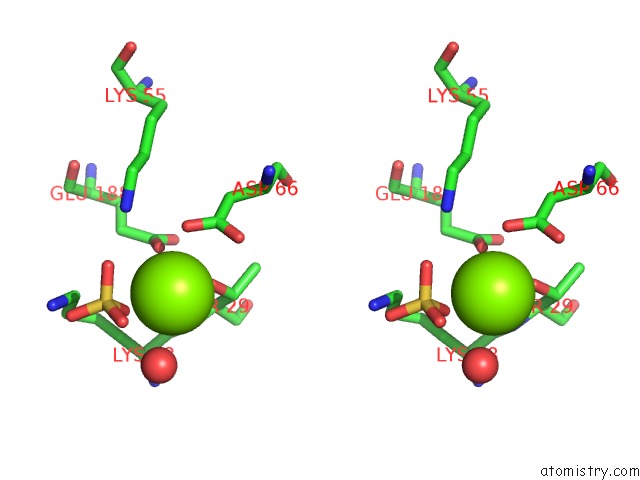
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Bifunctional Dapa Aminotransferase-Dtb Synthetase From Arabidopsis Thaliana in Its Apo Form. within 5.0Å range:
|
Reference:
D.Cobessi,
R.Dumas,
V.Pautre,
C.Meinguet,
J.L.Ferrer,
C.Alban.
Biochemical and Structural Characterization of the Arabidopsis Bifunctional Enzyme Dethiobiotin Synthetase- Diaminopelargonic Acid Aminotransferase: Evidence For Substrate Channeling in Biotin Synthesis. Plant Cell V. 24 1608 2012.
ISSN: ISSN 1040-4651
PubMed: 22547782
DOI: 10.1105/TPC.112.097675
Page generated: Mon Aug 11 05:30:18 2025
ISSN: ISSN 1040-4651
PubMed: 22547782
DOI: 10.1105/TPC.112.097675
Last articles
Mg in 4RRIMg in 4RRF
Mg in 4RRH
Mg in 4RRD
Mg in 4RRA
Mg in 4RR9
Mg in 4RR8
Mg in 4RR7
Mg in 4RQ5
Mg in 4RQI