Magnesium »
PDB 4f71-4fig »
4f8e »
Magnesium in PDB 4f8e: Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate
Enzymatic activity of Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate
All present enzymatic activity of Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate:
2.3.1.54;
2.3.1.54;
Protein crystallography data
The structure of Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate, PDB code: 4f8e
was solved by
A.Becker,
W.Kabsch,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 15 / 1.75 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.938, 153.169, 205.909, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.8 / 17.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate
(pdb code 4f8e). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate, PDB code: 4f8e:
In total only one binding site of Magnesium was determined in the Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate, PDB code: 4f8e:
Magnesium binding site 1 out of 1 in 4f8e
Go back to
Magnesium binding site 1 out
of 1 in the Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate
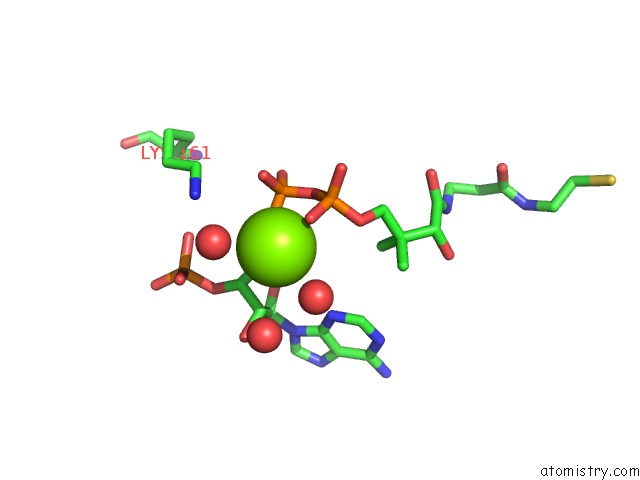
Mono view
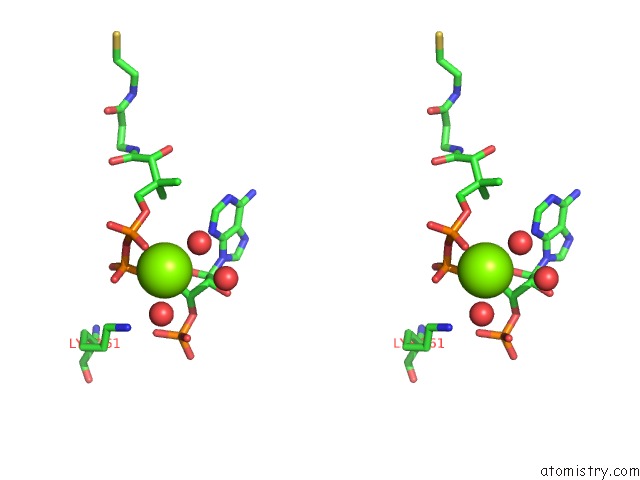
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Pyruvate Formate-Lyase (E.Coli) in Complex with Coa and the Substrate Analog Oxamate within 5.0Å range:
|
Reference:
A.Becker,
W.Kabsch.
X-Ray Structure of Pyruvate Formate-Lyase in Complex with Pyruvate and Coa.How the Enzyme Uses the Cys-418 Thiyl Radical For Pyruvate Cleavage J.Biol.Chem. V. 277 40036 2002.
ISSN: ISSN 0021-9258
PubMed: 12163496
DOI: 10.1074/JBC.M205821200
Page generated: Fri Aug 16 14:44:52 2024
ISSN: ISSN 0021-9258
PubMed: 12163496
DOI: 10.1074/JBC.M205821200
Last articles
Cl in 7T80Cl in 7T8J
Cl in 7T88
Cl in 7T4W
Cl in 7T4V
Cl in 7T7K
Cl in 7T6S
Cl in 7T6C
Cl in 7T4U
Cl in 7T4T