Magnesium »
PDB 4fk1-4fs5 »
4fme »
Magnesium in PDB 4fme: Espg-RAB1-ARF6 Complex
Protein crystallography data
The structure of Espg-RAB1-ARF6 Complex, PDB code: 4fme
was solved by
F.Shao,
Y.Zhu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 4.10 |
| Space group | P 43 |
| Cell size a, b, c (Å), α, β, γ (°) | 137.222, 137.222, 126.503, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.8 / 24.9 |
Other elements in 4fme:
The structure of Espg-RAB1-ARF6 Complex also contains other interesting chemical elements:
| Aluminium | (Al) | 2 atoms |
| Fluorine | (F) | 6 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Espg-RAB1-ARF6 Complex
(pdb code 4fme). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Espg-RAB1-ARF6 Complex, PDB code: 4fme:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Espg-RAB1-ARF6 Complex, PDB code: 4fme:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 4fme
Go back to
Magnesium binding site 1 out
of 4 in the Espg-RAB1-ARF6 Complex
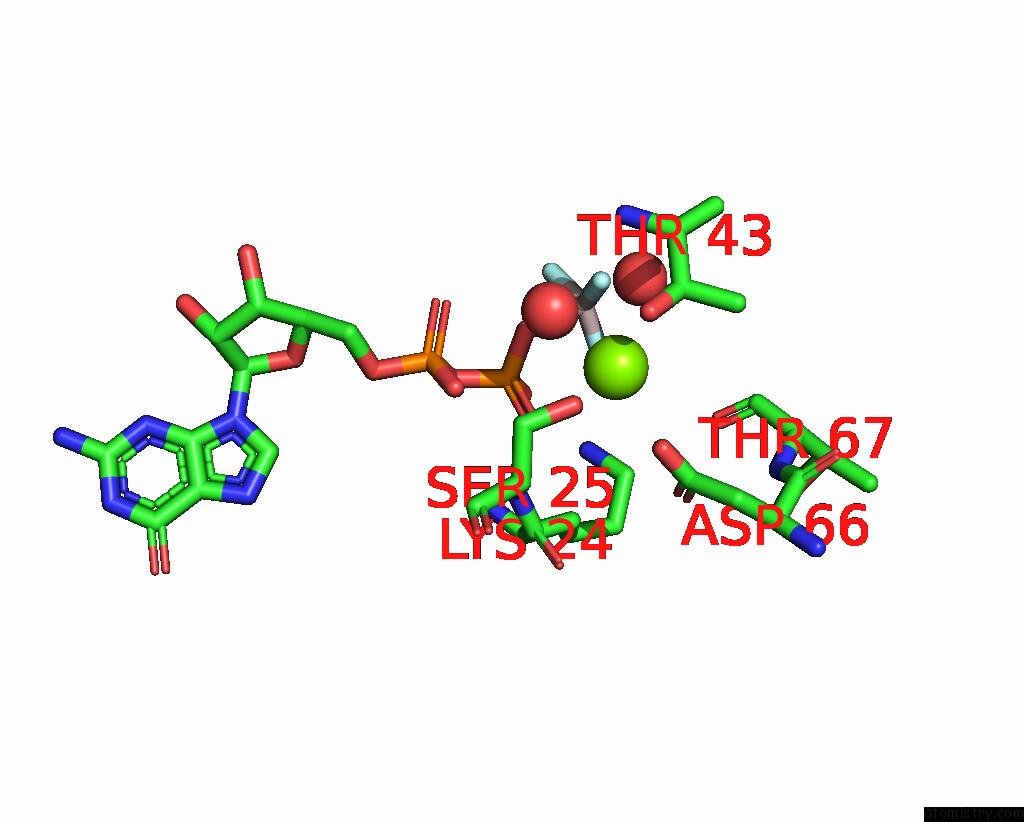
Mono view
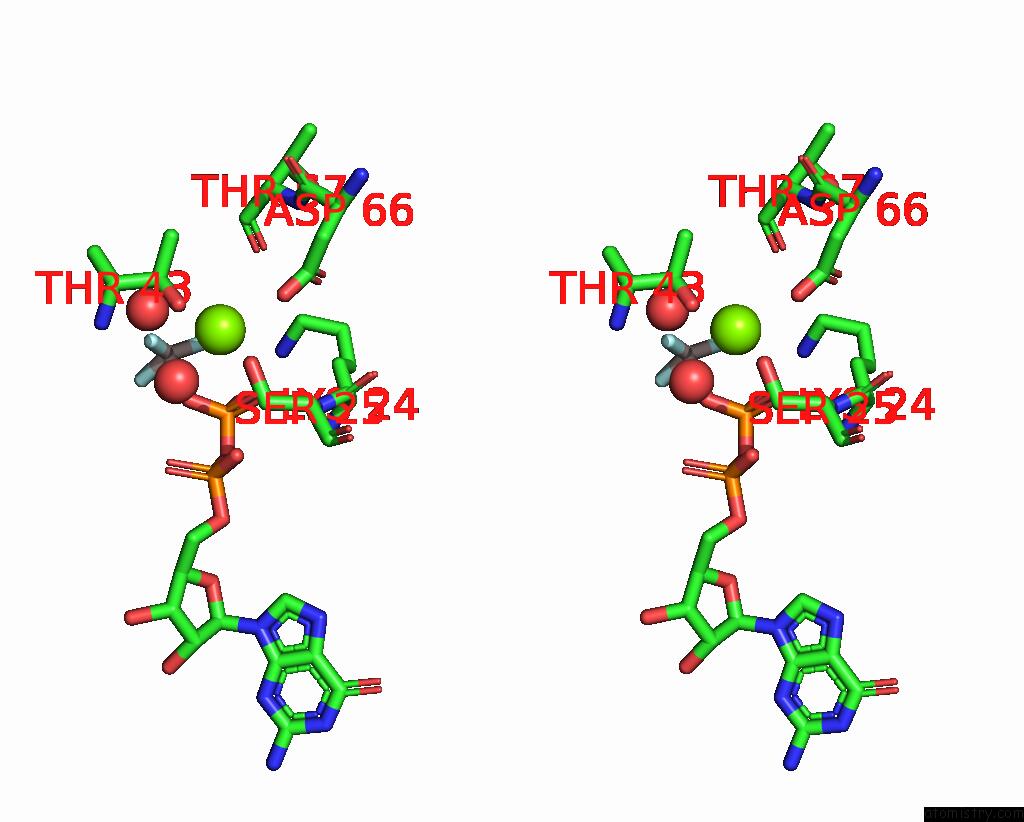
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Espg-RAB1-ARF6 Complex within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 4fme
Go back to
Magnesium binding site 2 out
of 4 in the Espg-RAB1-ARF6 Complex
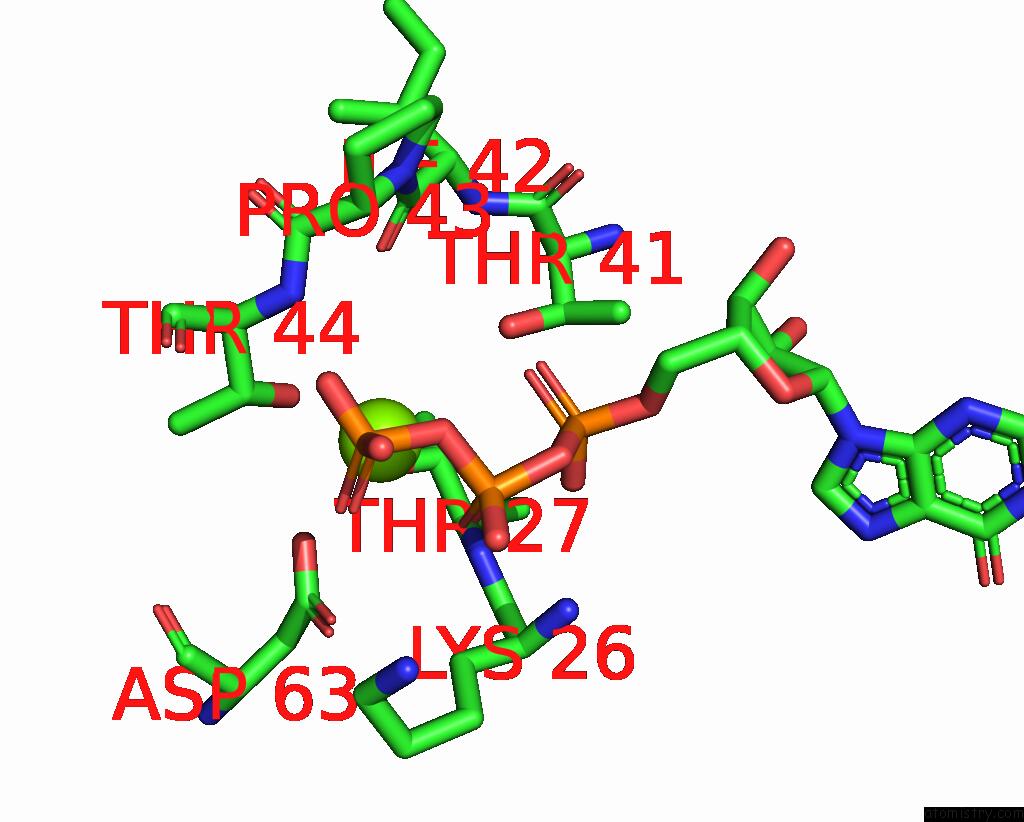
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Espg-RAB1-ARF6 Complex within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 4fme
Go back to
Magnesium binding site 3 out
of 4 in the Espg-RAB1-ARF6 Complex
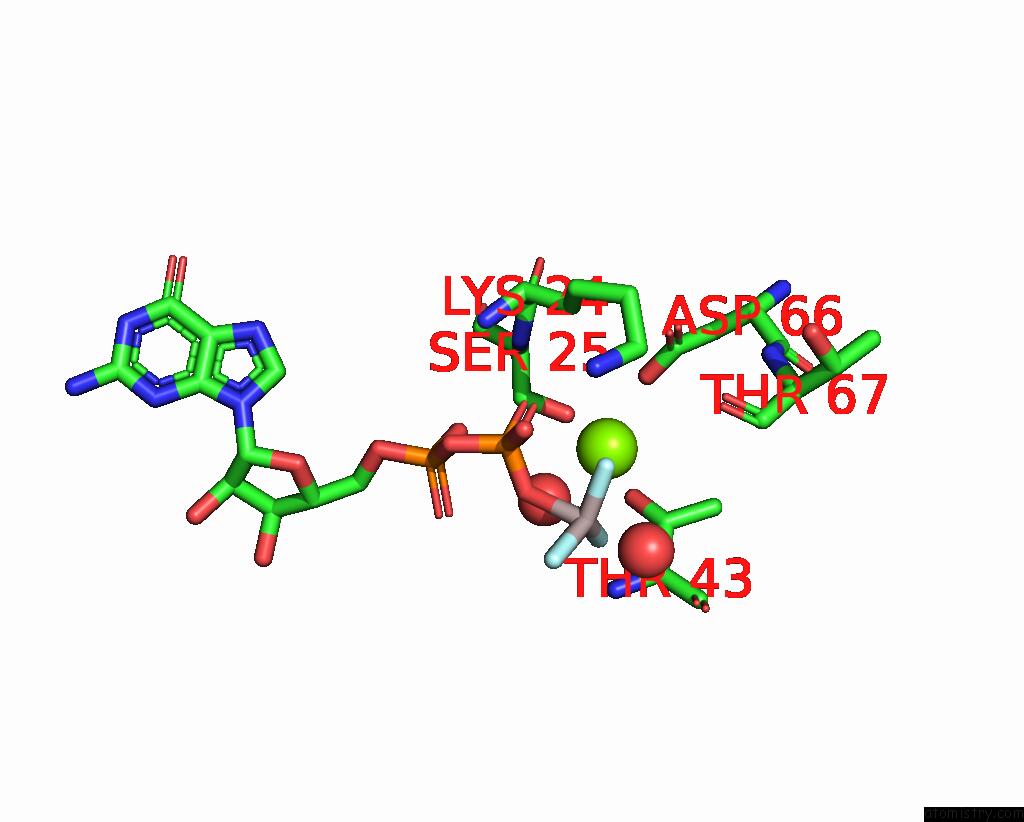
Mono view
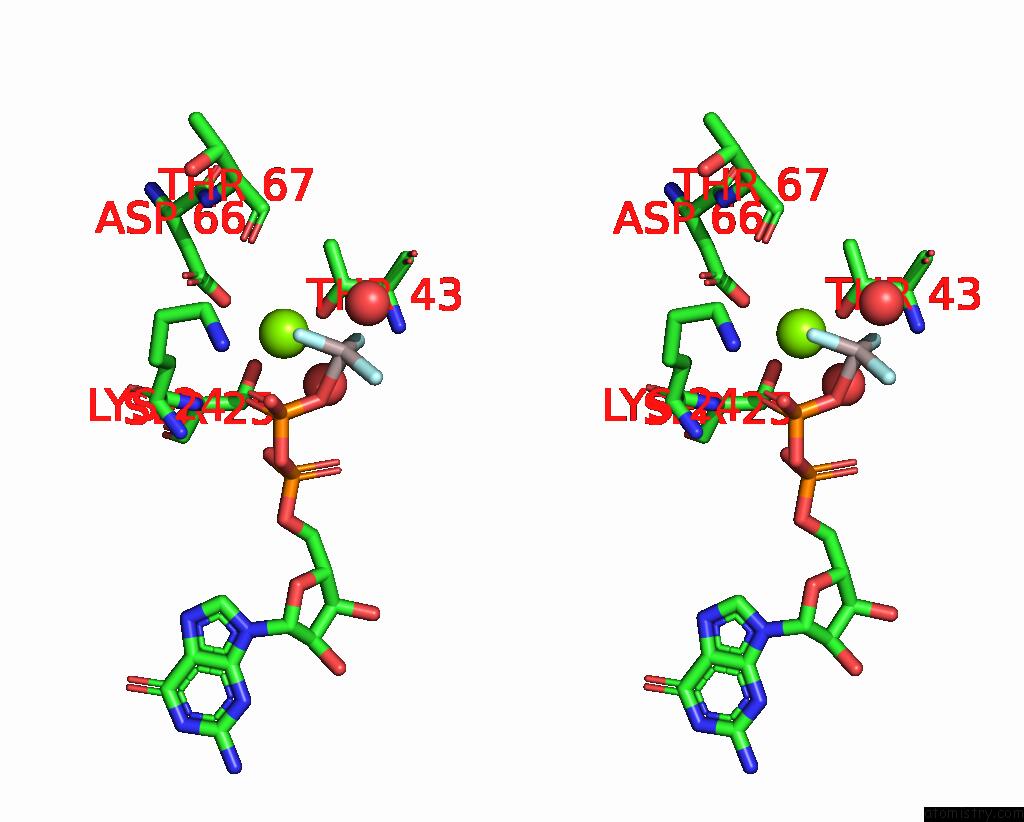
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Espg-RAB1-ARF6 Complex within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 4fme
Go back to
Magnesium binding site 4 out
of 4 in the Espg-RAB1-ARF6 Complex

Mono view
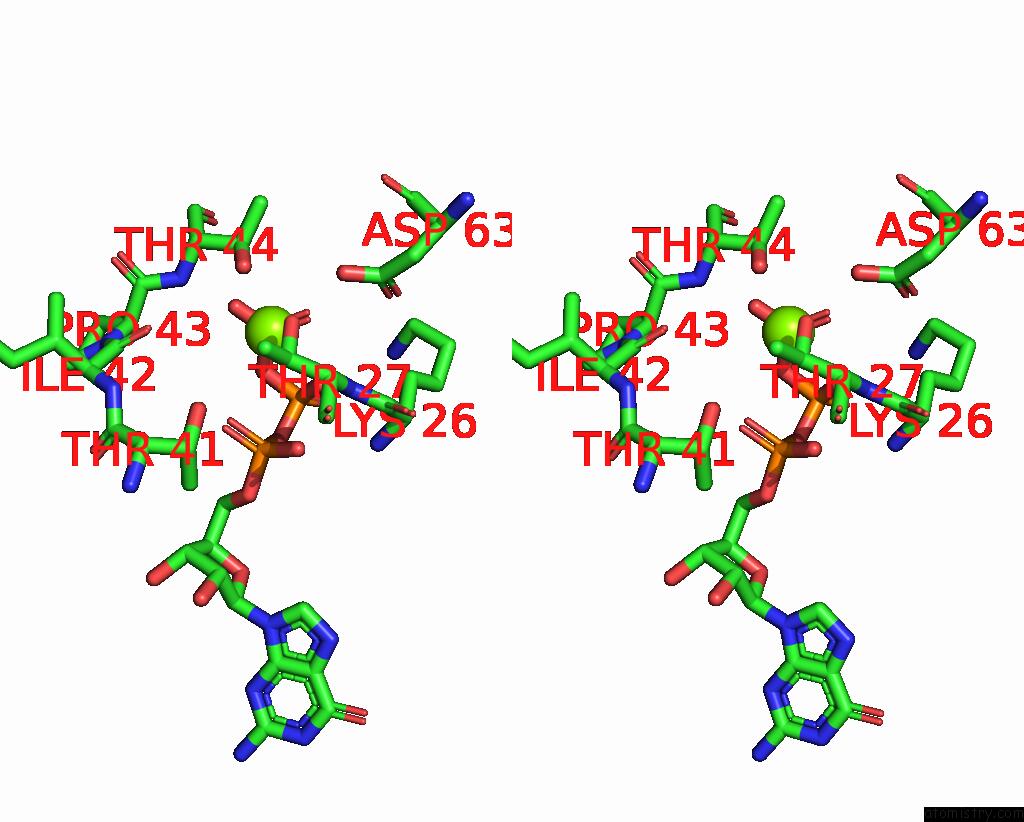
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Espg-RAB1-ARF6 Complex within 5.0Å range:
|
Reference:
N.Dong,
Y.Zhu,
Q.Lu,
L.Hu,
Y.Zheng,
F.Shao.
Structurally Distinct Bacterial Tbc-Like Gaps Link Arf Gtpase to RAB1 Inactivation to Counteract Host Defenses. Cell(Cambridge,Mass.) V. 150 1029 2012.
ISSN: ISSN 0092-8674
PubMed: 22939626
DOI: 10.1016/J.CELL.2012.06.050
Page generated: Mon Aug 11 12:59:28 2025
ISSN: ISSN 0092-8674
PubMed: 22939626
DOI: 10.1016/J.CELL.2012.06.050
Last articles
Mg in 4L9ZMg in 4L9Y
Mg in 4LA6
Mg in 4L9W
Mg in 4L81
Mg in 4L9S
Mg in 4L8N
Mg in 4L87
Mg in 4L8G
Mg in 4L80