Magnesium »
PDB 4fk1-4fs5 »
4fpp »
Magnesium in PDB 4fpp: Bacterial Phosphotransferase
Protein crystallography data
The structure of Bacterial Phosphotransferase, PDB code: 4fpp
was solved by
A.Fioravanti,
B.Clantin,
F.Dewitte,
Z.Lens,
A.Verger,
E.Biondi,
V.Villeret,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.97 / 2.20 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 103.540, 210.160, 93.930, 90.00, 90.00, 90.00 |
| R / Rfree (%) | n/a / 26.4 |
Other elements in 4fpp:
The structure of Bacterial Phosphotransferase also contains other interesting chemical elements:
| Nickel | (Ni) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Bacterial Phosphotransferase
(pdb code 4fpp). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Bacterial Phosphotransferase, PDB code: 4fpp:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Bacterial Phosphotransferase, PDB code: 4fpp:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 4fpp
Go back to
Magnesium binding site 1 out
of 3 in the Bacterial Phosphotransferase
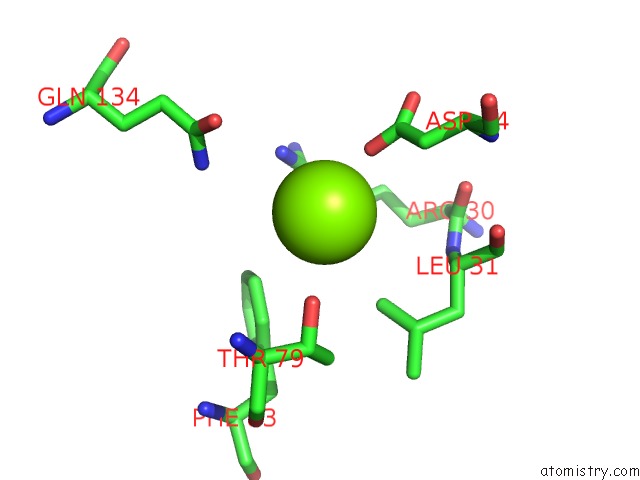
Mono view
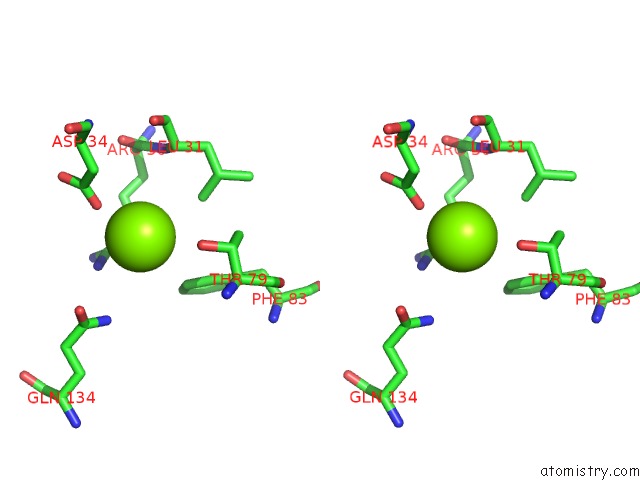
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Bacterial Phosphotransferase within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 4fpp
Go back to
Magnesium binding site 2 out
of 3 in the Bacterial Phosphotransferase
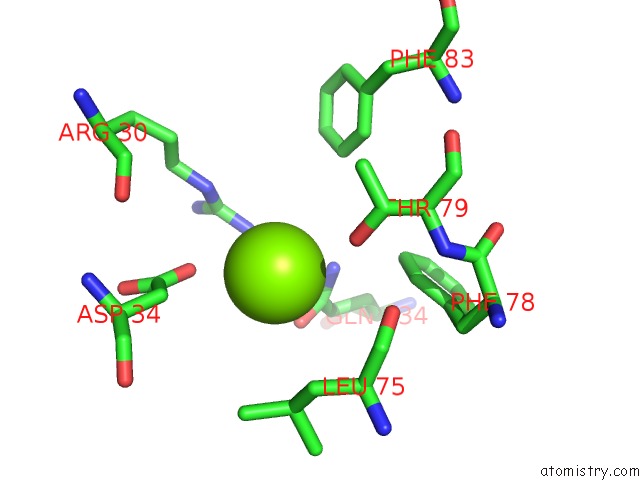
Mono view
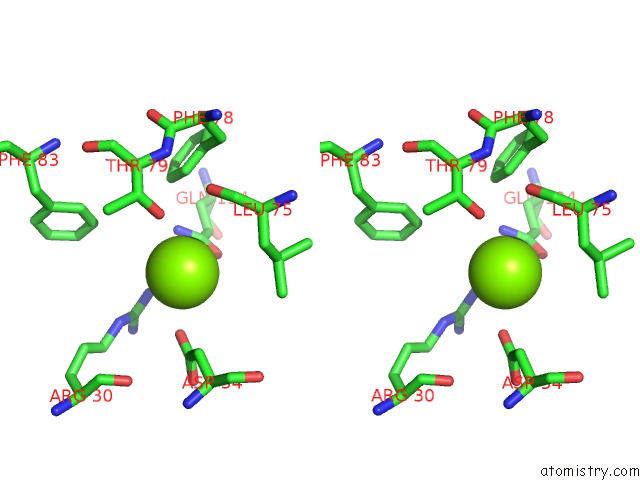
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Bacterial Phosphotransferase within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 4fpp
Go back to
Magnesium binding site 3 out
of 3 in the Bacterial Phosphotransferase
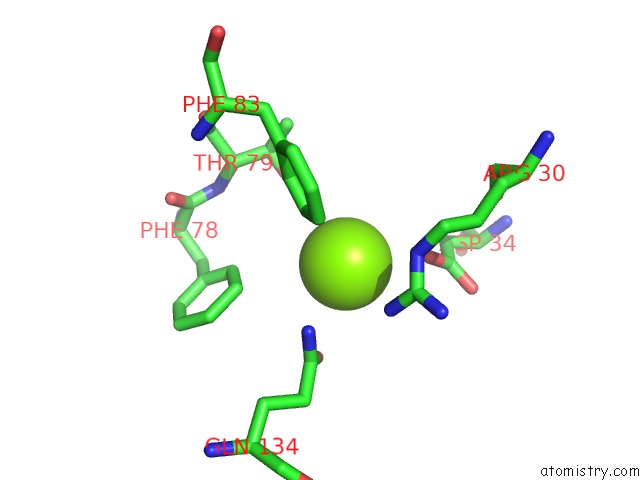
Mono view
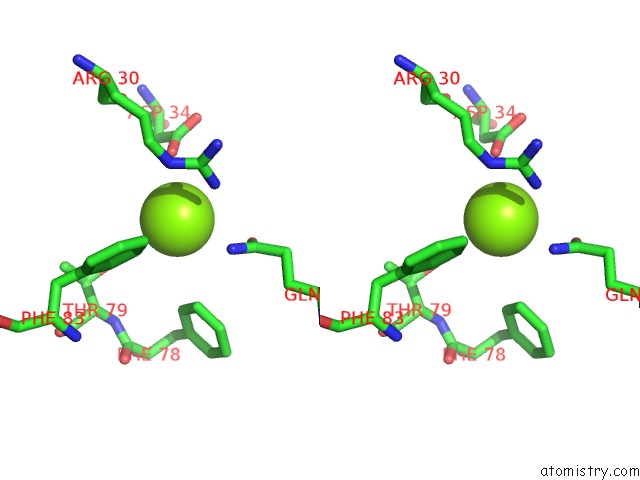
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Bacterial Phosphotransferase within 5.0Å range:
|
Reference:
A.Fioravanti,
B.Clantin,
F.Dewitte,
Z.Lens,
A.Verger,
E.G.Biondi,
V.Villeret.
Structural Insights Into Chpt, An Essential Dimeric Histidine Phosphotransferase Regulating the Cell Cycle in Caulobacter Crescentus. Acta Crystallogr.,Sect.F V. 68 1025 2012.
ISSN: ESSN 1744-3091
PubMed: 22949187
DOI: 10.1107/S1744309112033064
Page generated: Fri Aug 16 15:13:57 2024
ISSN: ESSN 1744-3091
PubMed: 22949187
DOI: 10.1107/S1744309112033064
Last articles
F in 4F5NF in 4F4Q
F in 4F4P
F in 4F2A
F in 4F2Y
F in 4E99
F in 4F2X
F in 4EZJ
F in 4EWS
F in 4ELF