Magnesium »
PDB 4hyw-4iaj »
4i9g »
Magnesium in PDB 4i9g: Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium
Protein crystallography data
The structure of Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium, PDB code: 4i9g
was solved by
T.Biswas,
G.Larrouy-Maumus,
L.P.De Carvalho,
O.V.Tsodikov,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 3.25 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.368, 71.196, 94.621, 90.00, 105.52, 90.00 |
| R / Rfree (%) | 22 / 27.2 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium
(pdb code 4i9g). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium, PDB code: 4i9g:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium, PDB code: 4i9g:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4i9g
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium
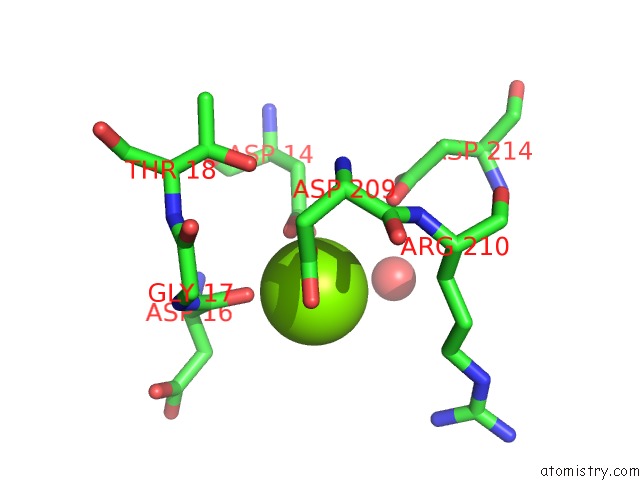
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4i9g
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Glycerol Phosphate Phosphatase RV1692 From Mycobacterium Tuberculosis in Complex with Magnesium within 5.0Å range:
|
Reference:
G.Larrouy-Maumus,
T.Biswas,
D.M.Hunt,
G.Kelly,
O.V.Tsodikov,
L.P.De Carvalho.
Discovery of A Glycerol 3-Phosphate Phosphatase Reveals Glycerophospholipid Polar Head Recycling in Mycobacterium Tuberculosis. Proc.Natl.Acad.Sci.Usa V. 110 11320 2013.
ISSN: ISSN 0027-8424
PubMed: 23801751
DOI: 10.1073/PNAS.1221597110
Page generated: Mon Aug 11 14:06:25 2025
ISSN: ISSN 0027-8424
PubMed: 23801751
DOI: 10.1073/PNAS.1221597110
Last articles
Mg in 4LF9Mg in 4LF7
Mg in 4LF6
Mg in 4LF4
Mg in 4LF5
Mg in 4LCZ
Mg in 4LF2
Mg in 4LF1
Mg in 4LEM
Mg in 4LCK