Magnesium »
PDB 4kg0-4knx »
4kjd »
Magnesium in PDB 4kjd: Ratintestinal Ap Expressed in E. Coli
Enzymatic activity of Ratintestinal Ap Expressed in E. Coli
All present enzymatic activity of Ratintestinal Ap Expressed in E. Coli:
3.1.3.1;
3.1.3.1;
Protein crystallography data
The structure of Ratintestinal Ap Expressed in E. Coli, PDB code: 4kjd
was solved by
K.Ghosh,
R.K.Anumula,
B.K.Laksmaiah,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.68 / 2.21 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 63.321, 75.726, 170.710, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.7 / 20.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Ratintestinal Ap Expressed in E. Coli
(pdb code 4kjd). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Ratintestinal Ap Expressed in E. Coli, PDB code: 4kjd:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Ratintestinal Ap Expressed in E. Coli, PDB code: 4kjd:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 4kjd
Go back to
Magnesium binding site 1 out
of 4 in the Ratintestinal Ap Expressed in E. Coli

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Ratintestinal Ap Expressed in E. Coli within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 4kjd
Go back to
Magnesium binding site 2 out
of 4 in the Ratintestinal Ap Expressed in E. Coli

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Ratintestinal Ap Expressed in E. Coli within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 4kjd
Go back to
Magnesium binding site 3 out
of 4 in the Ratintestinal Ap Expressed in E. Coli
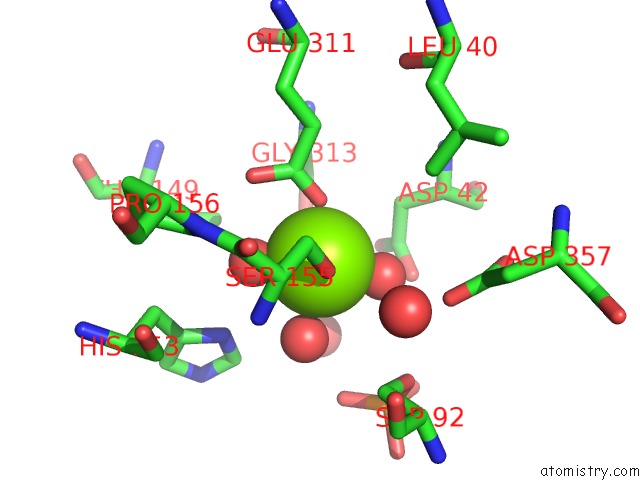
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Ratintestinal Ap Expressed in E. Coli within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 4kjd
Go back to
Magnesium binding site 4 out
of 4 in the Ratintestinal Ap Expressed in E. Coli

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Ratintestinal Ap Expressed in E. Coli within 5.0Å range:
|
Reference:
K.Ghosh,
D.Mazumder Tagore,
R.Anumula,
B.Lakshmaiah,
P.P.Kumar,
S.Singaram,
T.Matan,
S.Kallipatti,
S.Selvam,
P.Krishnamurthy,
M.Ramarao.
Crystal Structure of Rat Intestinal Alkaline Phosphatase - Role of Crown Domain in Mammalian Alkaline Phosphatases. J.Struct.Biol. V. 184 182 2013.
ISSN: ISSN 1047-8477
PubMed: 24076154
DOI: 10.1016/J.JSB.2013.09.017
Page generated: Sat Aug 17 03:39:52 2024
ISSN: ISSN 1047-8477
PubMed: 24076154
DOI: 10.1016/J.JSB.2013.09.017
Last articles
Cl in 5XVUCl in 5XVG
Cl in 5XVF
Cl in 5XVA
Cl in 5XT8
Cl in 5XUH
Cl in 5XUR
Cl in 5XU8
Cl in 5XUJ
Cl in 5XU7