Magnesium »
PDB 4lja-4lsh »
4lrt »
Magnesium in PDB 4lrt: Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site
Enzymatic activity of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site
All present enzymatic activity of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site:
1.2.1.10; 4.1.3.39;
1.2.1.10; 4.1.3.39;
Protein crystallography data
The structure of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site, PDB code: 4lrt
was solved by
B.Fischer,
G.Branlant,
F.Talfournier,
A.Gruez,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.45 / 1.50 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 94.500, 116.700, 131.500, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.1 / 17 |
Other elements in 4lrt:
The structure of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site also contains other interesting chemical elements:
| Sodium | (Na) | 4 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site
(pdb code 4lrt). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site, PDB code: 4lrt:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site, PDB code: 4lrt:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4lrt
Go back to
Magnesium binding site 1 out
of 2 in the Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site
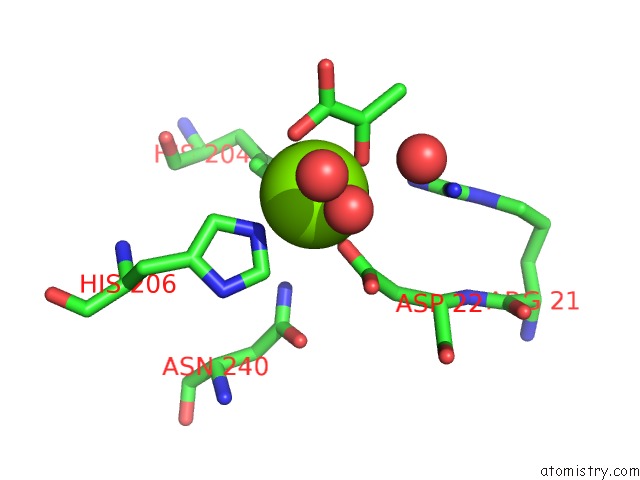
Mono view
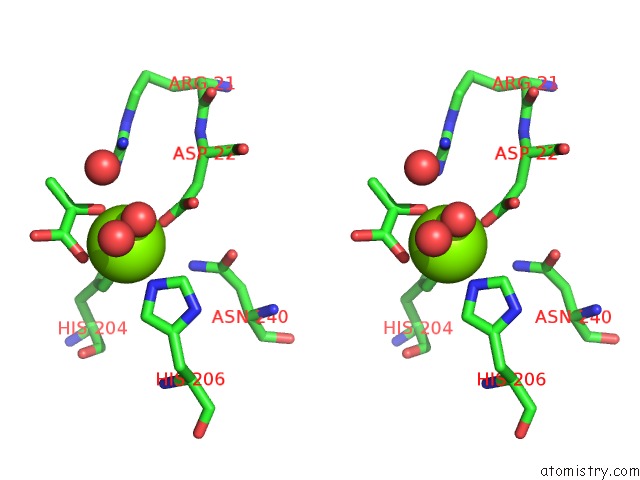
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4lrt
Go back to
Magnesium binding site 2 out
of 2 in the Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site
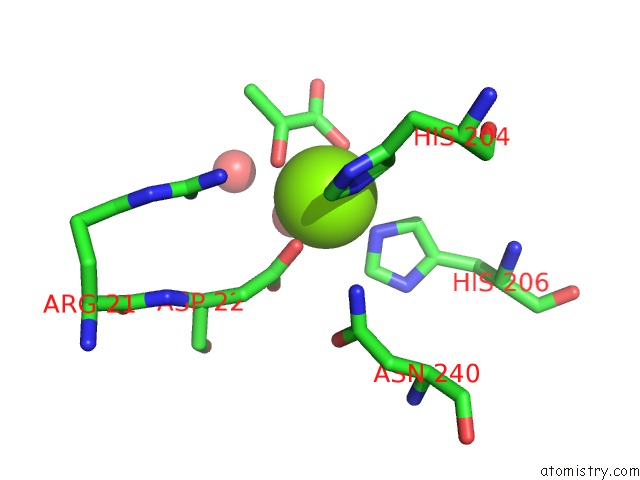
Mono view
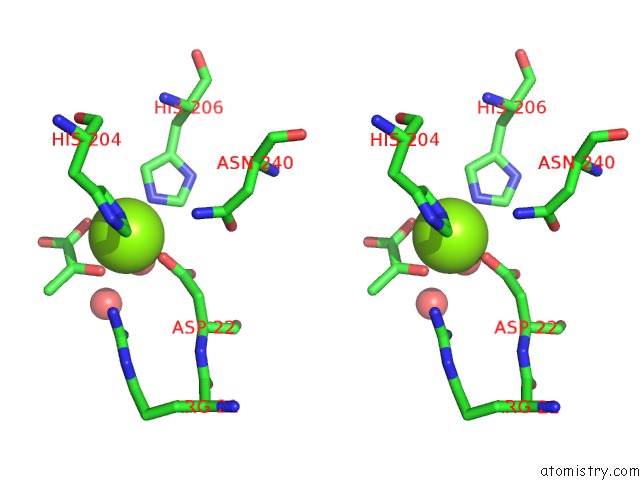
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site within 5.0Å range:
|
Reference:
B.Fischer,
G.Branlant,
F.Talfournier,
A.Gruez.
Crystal and Solution Structures of the Bifunctional Enzyme (Aldolase/Aldehyde Dehydrogenase) From Thermomonospora Curvata, Reveal A Cofactor-Binding Domain Motion During Nad+ and Coa Accommodation Whithin the Shared Cofactor-Binding Site To Be Published.
Page generated: Mon Aug 11 20:07:12 2025
Last articles
Mg in 4RL3Mg in 4RML
Mg in 4RKQ
Mg in 4RJK
Mg in 4RKD
Mg in 4RKF
Mg in 4RJJ
Mg in 4RKE
Mg in 4RKC
Mg in 4RGE