Magnesium »
PDB 4lsi-4m35 »
4m2z »
Magnesium in PDB 4m2z: Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
Enzymatic activity of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
All present enzymatic activity of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage):
3.1.26.3;
3.1.26.3;
Protein crystallography data
The structure of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage), PDB code: 4m2z
was solved by
J.Gan,
Y.-H.Liang,
G.X.Shaw,
J.E.Tropea,
D.S.Waugh,
X.Ji,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.11 / 2.85 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 81.131, 81.131, 223.892, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 22.1 / 28.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
(pdb code 4m2z). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage), PDB code: 4m2z:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage), PDB code: 4m2z:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 4m2z
Go back to
Magnesium binding site 1 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
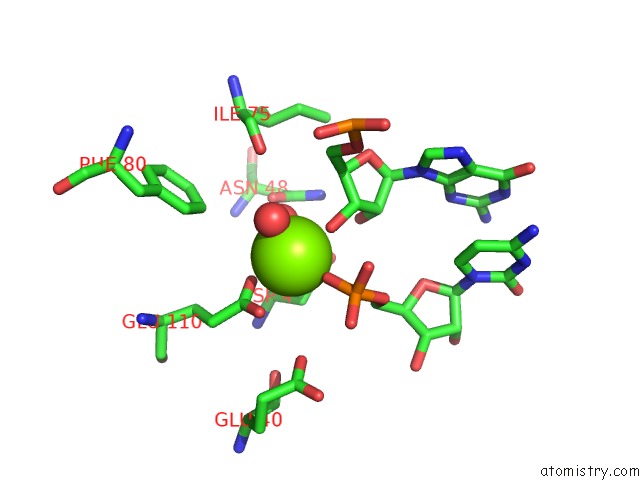
Mono view
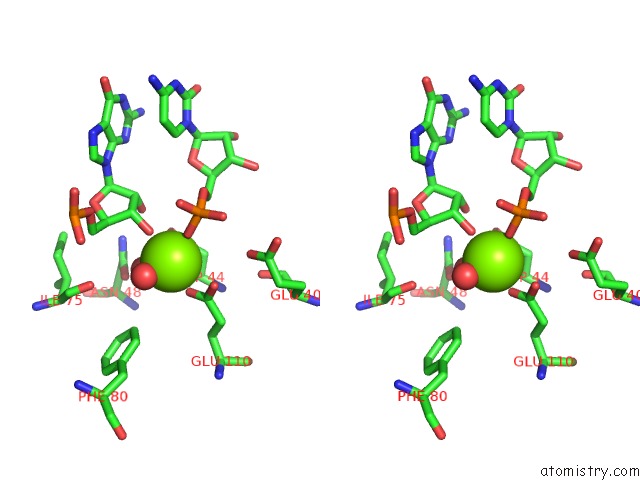
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 4m2z
Go back to
Magnesium binding site 2 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
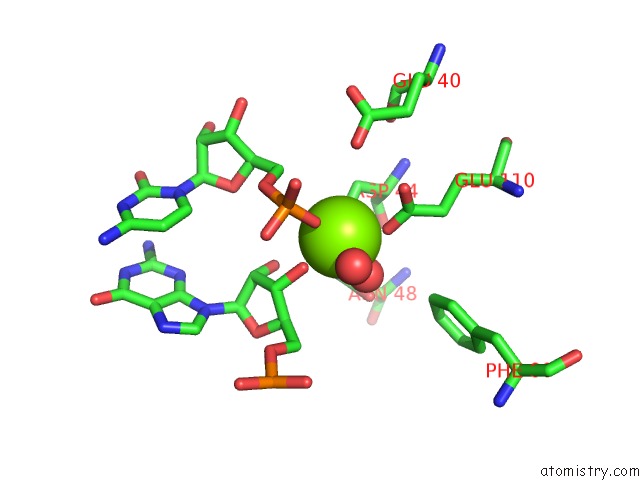
Mono view
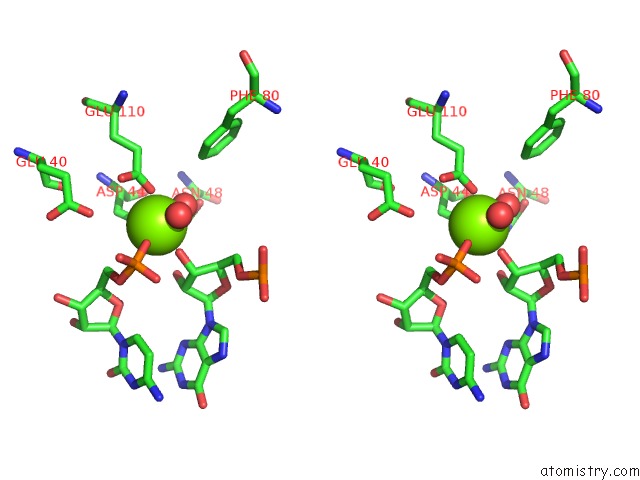
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 4m2z
Go back to
Magnesium binding site 3 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
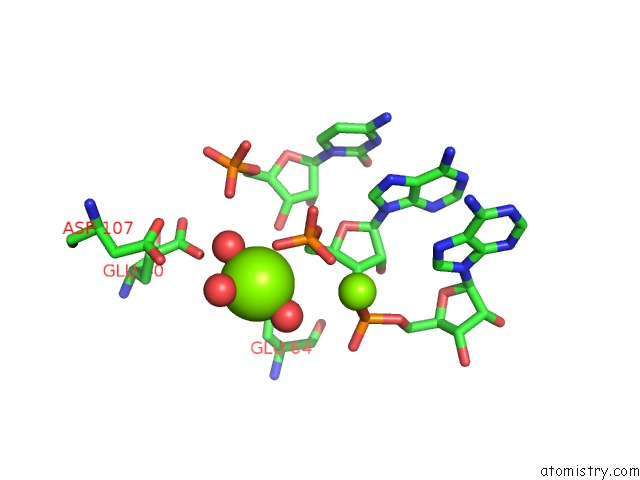
Mono view
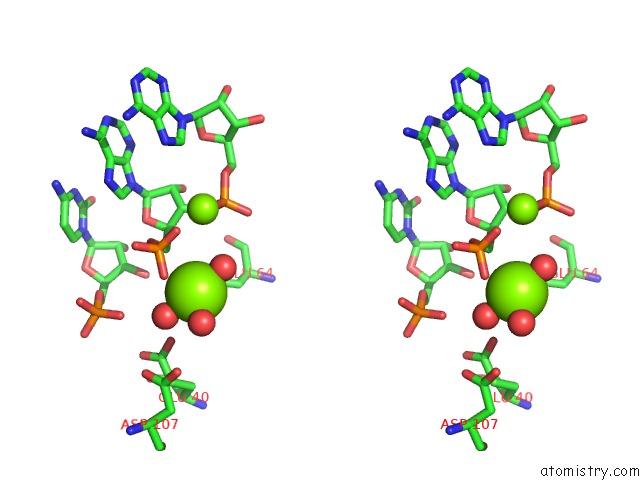
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 4m2z
Go back to
Magnesium binding site 4 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
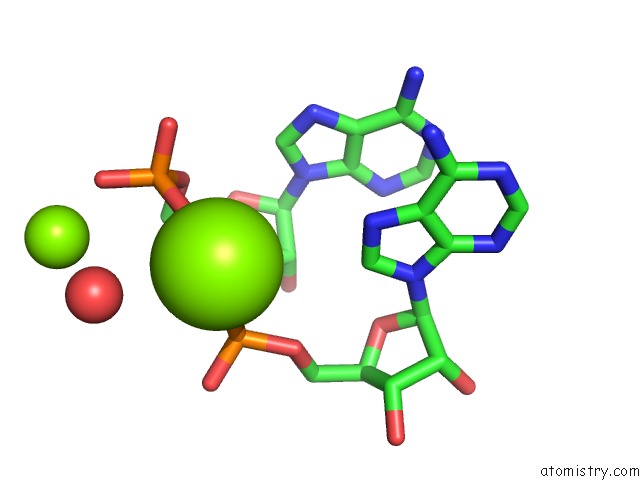
Mono view
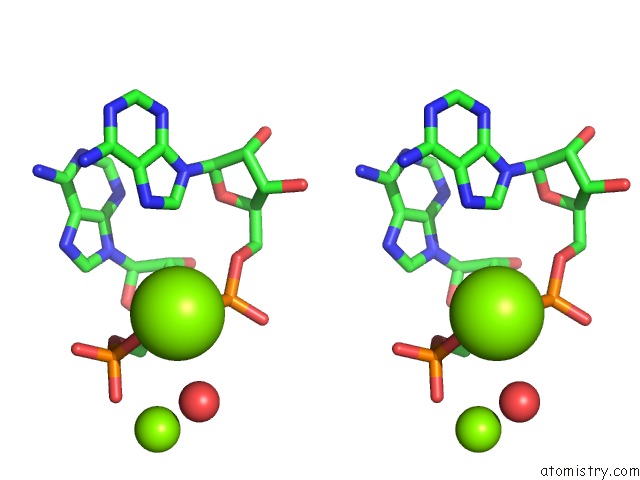
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 4m2z
Go back to
Magnesium binding site 5 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
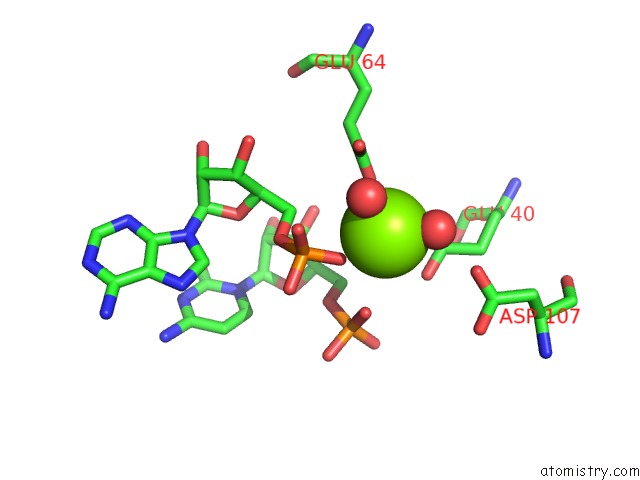
Mono view
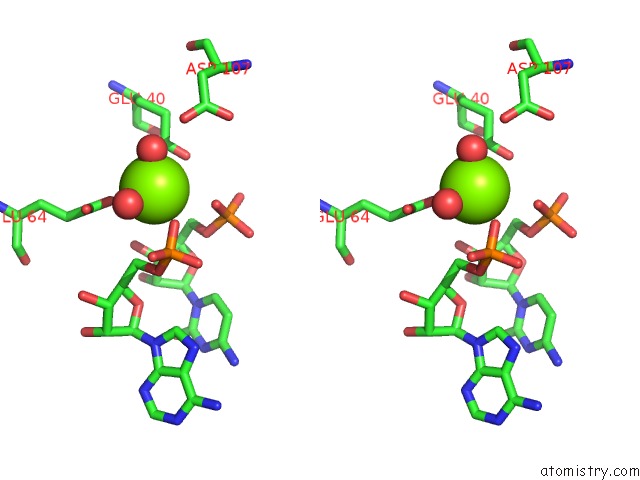
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 4m2z
Go back to
Magnesium binding site 6 out
of 6 in the Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage)
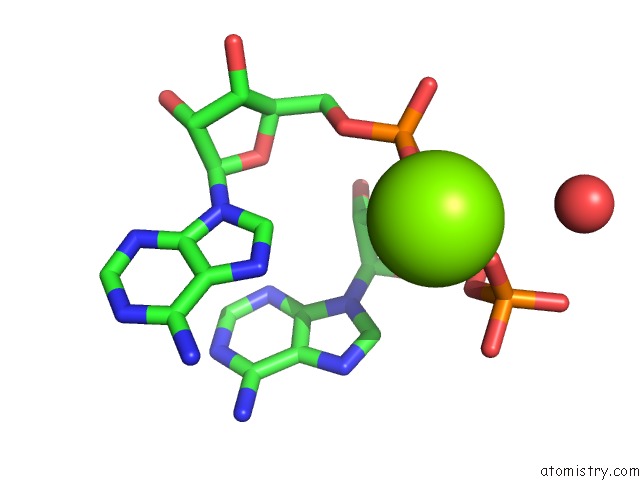
Mono view
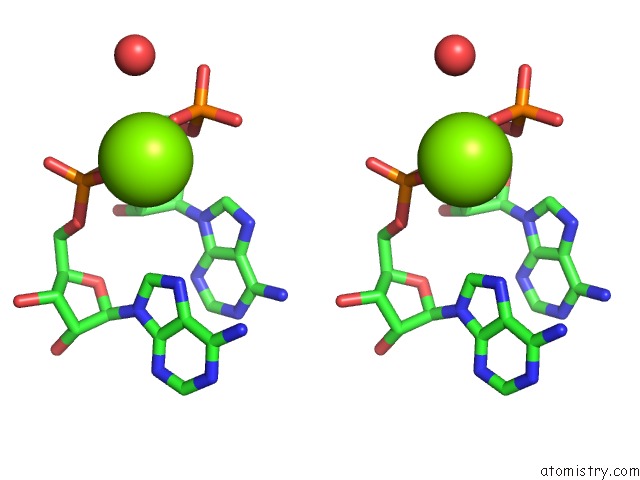
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Rnase III Complexed with Double-Stranded Rna and Cmp (Type II Cleavage) within 5.0Å range:
|
Reference:
D.L.Court,
J.Gan,
Y.H.Liang,
G.X.Shaw,
J.E.Tropea,
N.Costantino,
D.S.Waugh,
X.Ji.
Rnase III: Genetics and Function; Structure and Mechanism. Annu. Rev. Genet. V. 47 405 2013.
PubMed: 24274754
DOI: 10.1146/ANNUREV-GENET-110711-155618
Page generated: Mon Aug 19 20:14:47 2024
PubMed: 24274754
DOI: 10.1146/ANNUREV-GENET-110711-155618
Last articles
F in 4HXNF in 4HT2
F in 4HU1
F in 4HVS
F in 4HW7
F in 4HUA
F in 4HU9
F in 4HQJ
F in 4HT3
F in 4HLQ