Magnesium »
PDB 4m3n-4mfe »
4maz »
Magnesium in PDB 4maz: The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus
Enzymatic activity of The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus
All present enzymatic activity of The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus:
3.2.1.10;
3.2.1.10;
Protein crystallography data
The structure of The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus, PDB code: 4maz
was solved by
J.K.Hobbs,
W.Jiao,
A.D.Easter,
E.J.Parker,
L.A.Schipper,
V.L.Arcus,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.69 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.470, 100.710, 61.440, 90.00, 112.77, 90.00 |
| R / Rfree (%) | 16.1 / 18.8 |
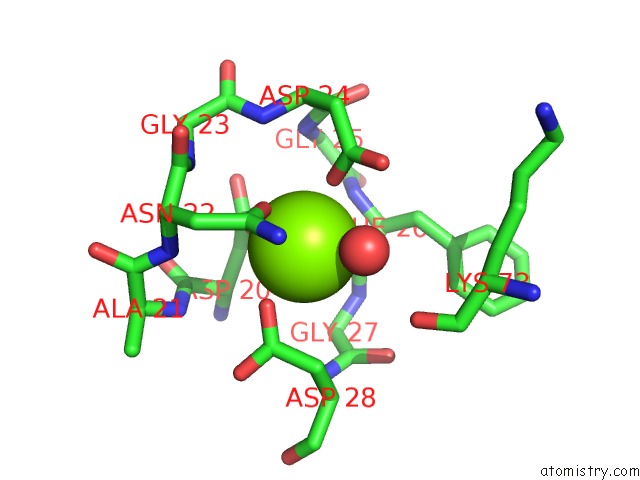
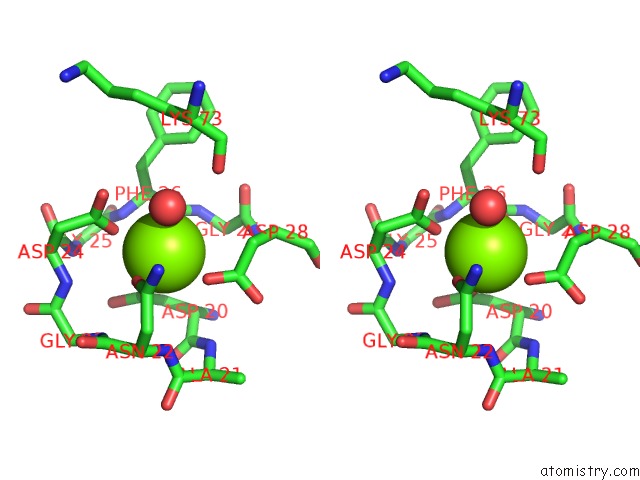
Magnesium Binding Sites:
The binding sites of Magnesium atom in the The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus
(pdb code 4maz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus, PDB code: 4maz:
In total only one binding site of Magnesium was determined in the The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus, PDB code: 4maz:
Magnesium binding site 1 out of 1 in 4maz
Go back to
Magnesium binding site 1 out
of 1 in the The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of The Structure of Mall Mutant Enzyme V200S From Bacillus Subtilus within 5.0Å range:
|
Reference:
J.K.Hobbs,
W.Jiao,
A.D.Easter,
E.J.Parker,
L.A.Schipper,
V.L.Arcus.
Change in Heat Capacity For Enzyme Catalysis Determines Temperature Dependence of Enzyme Catalyzed Rates. Acs Chem.Biol. V. 8 2388 2013.
ISSN: ISSN 1554-8929
PubMed: 24015933
DOI: 10.1021/CB4005029
Page generated: Mon Aug 11 20:21:17 2025
ISSN: ISSN 1554-8929
PubMed: 24015933
DOI: 10.1021/CB4005029
Last articles
Mg in 4UQLMg in 4UQO
Mg in 4UOO
Mg in 4UPH
Mg in 4UPV
Mg in 4UOP
Mg in 4UOG
Mg in 4UOH
Mg in 4UOF
Mg in 4UN5