Magnesium »
PDB 4rcz-4rkf »
4rix »
Magnesium in PDB 4rix: Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
Enzymatic activity of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
All present enzymatic activity of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation:
2.7.10.1;
2.7.10.1;
Protein crystallography data
The structure of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation, PDB code: 4rix
was solved by
P.Littlefield,
L.Liu,
N.Jura,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 59.77 / 3.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.649, 155.053, 86.857, 90.00, 111.09, 90.00 |
| R / Rfree (%) | 20.7 / 25.8 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
(pdb code 4rix). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation, PDB code: 4rix:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation, PDB code: 4rix:
Jump to Magnesium binding site number: 1; 2; 3; 4;
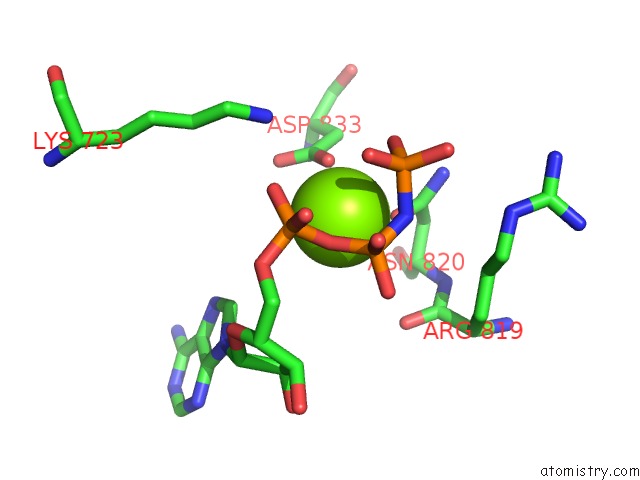
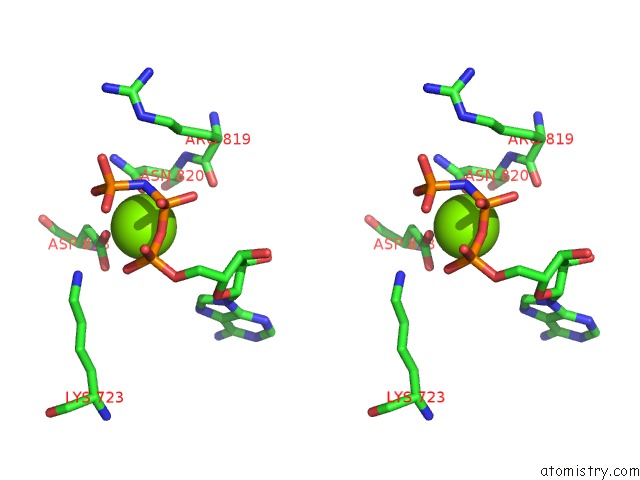
Magnesium binding site 1 out of 4 in 4rix
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation within 5.0Å range:
|
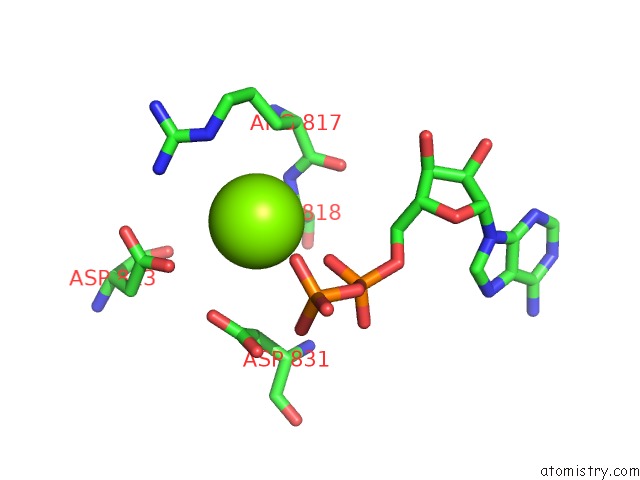
Magnesium binding site 2 out of 4 in 4rix
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation within 5.0Å range:
|
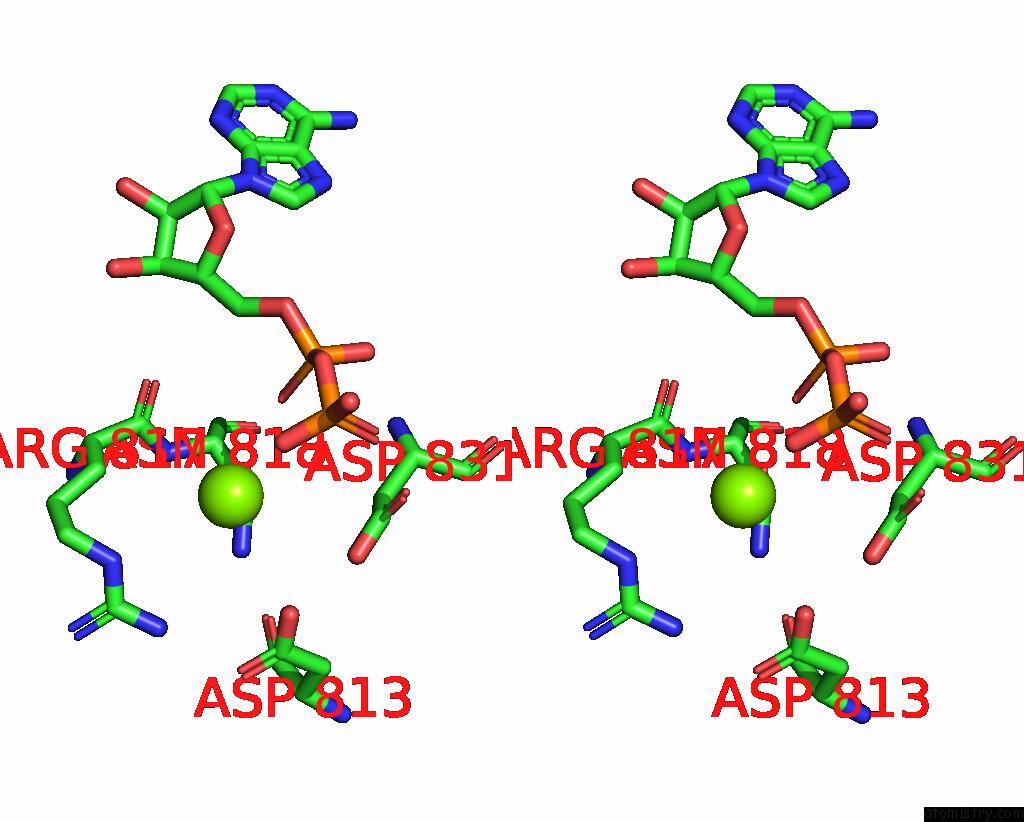
Magnesium binding site 3 out of 4 in 4rix
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
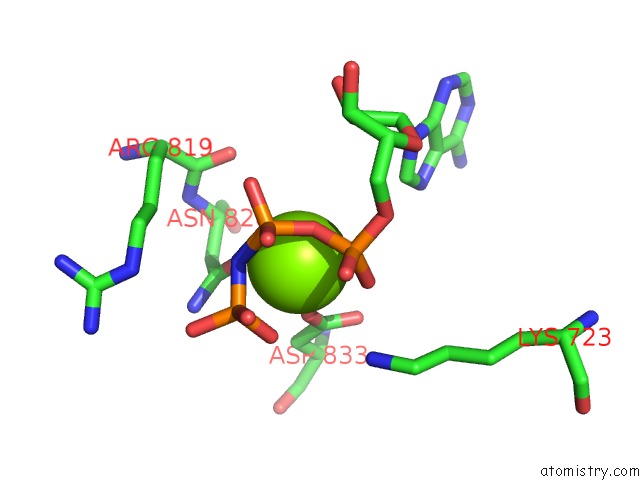
Mono view
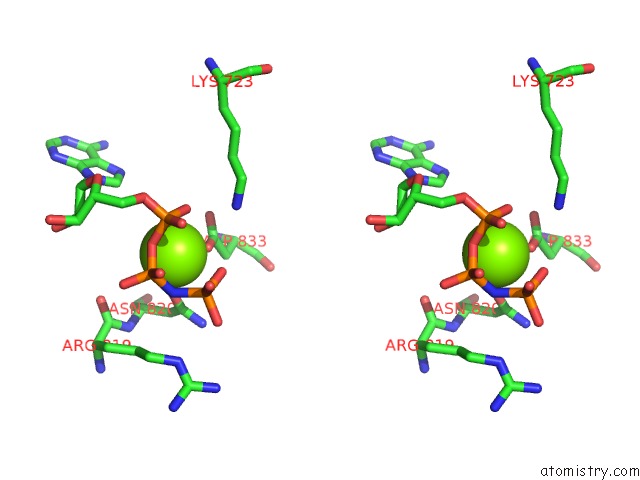
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 4rix
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation
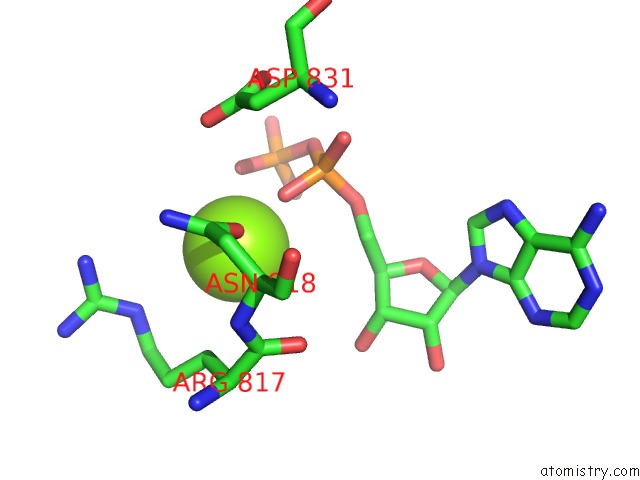
Mono view
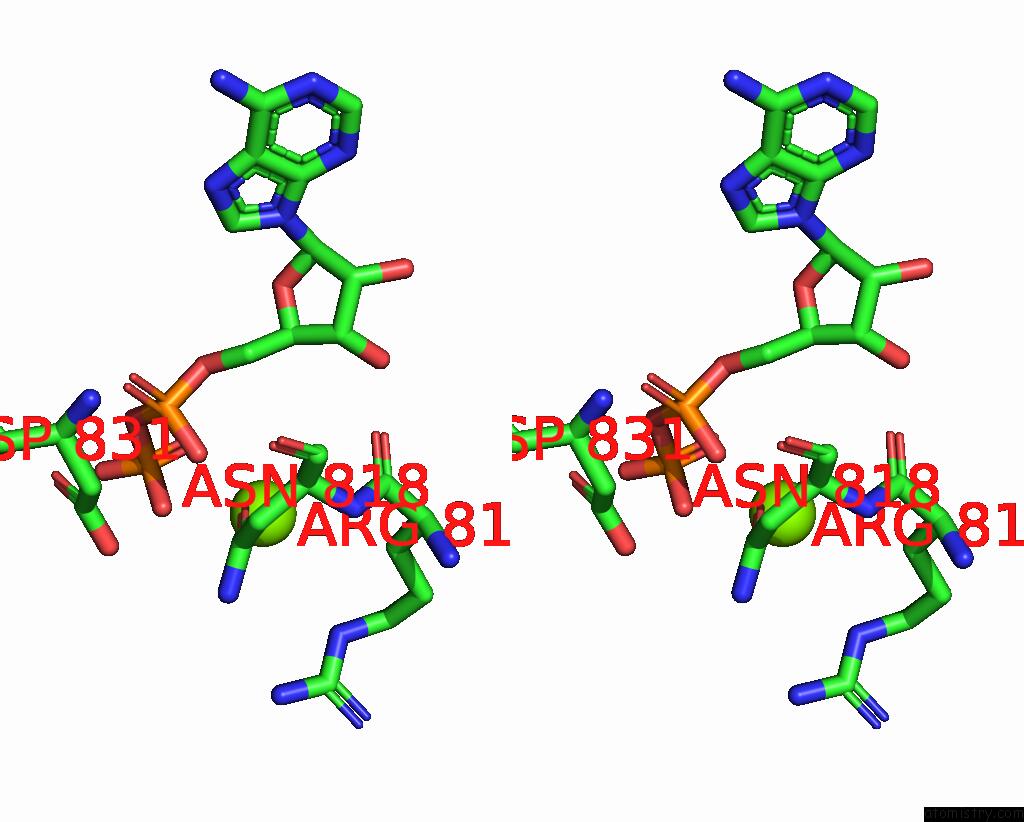
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of An Egfr/HER3 Kinase Domain Heterodimer Containing the Cancer-Associated HER3-Q790R Mutation within 5.0Å range:
|
Reference:
P.Littlefield,
L.Liu,
V.Mysore,
Y.Shan,
D.E.Shaw,
N.Jura.
Structural Analysis of the Egfr/HER3 Heterodimer Reveals the Molecular Basis For Activating HER3 Mutations. Sci.Signal. V. 7 RA114 2014.
ISSN: ESSN 1937-9145
PubMed: 25468994
DOI: 10.1126/SCISIGNAL.2005786
Page generated: Mon Aug 11 23:21:06 2025
ISSN: ESSN 1937-9145
PubMed: 25468994
DOI: 10.1126/SCISIGNAL.2005786
Last articles
Mg in 4XLPMg in 4XJC
Mg in 4XLN
Mg in 4XJ7
Mg in 4XKC
Mg in 4XJX
Mg in 4XJ6
Mg in 4XJ5
Mg in 4XIY
Mg in 4XJ3