Magnesium »
PDB 4rrk-4s1l »
4rsr »
Magnesium in PDB 4rsr: Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone
Protein crystallography data
The structure of Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone, PDB code: 4rsr
was solved by
C.Packianathan,
K.Marapakala,
A.A.Ajees,
P.Kandavelu,
B.P.Rosen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.70 / 2.25 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 85.156, 46.406, 100.255, 90.00, 114.07, 90.00 |
| R / Rfree (%) | 17.3 / 22.4 |
Other elements in 4rsr:
The structure of Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone also contains other interesting chemical elements:
| Arsenic | (As) | 1 atom |
| Calcium | (Ca) | 2 atoms |
| Sodium | (Na) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone
(pdb code 4rsr). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone, PDB code: 4rsr:
In total only one binding site of Magnesium was determined in the Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone, PDB code: 4rsr:
Magnesium binding site 1 out of 1 in 4rsr
Go back to
Magnesium binding site 1 out
of 1 in the Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone
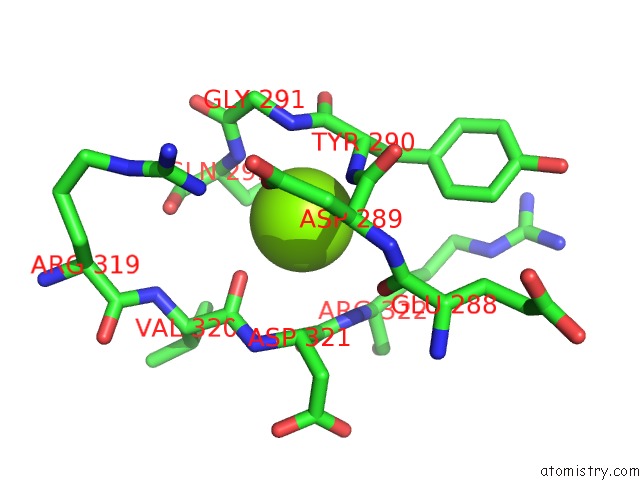
Mono view
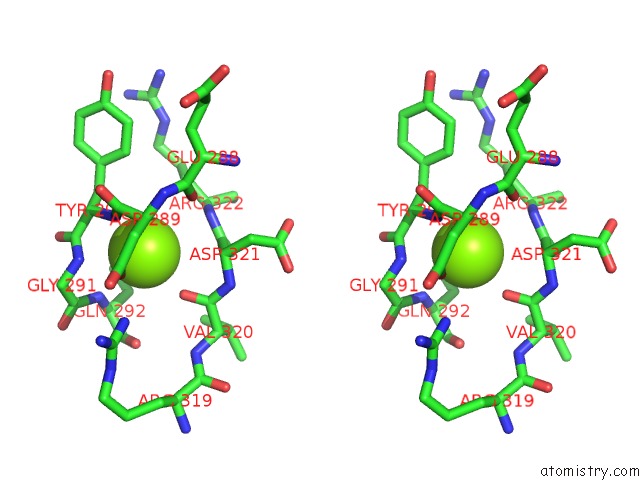
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Arsm Arsenic(III) S-Adenosylmethionine Methyltransferase with Trivalent Phenyl Arsencial Derivative-Roxarsone within 5.0Å range:
|
Reference:
C.Packianathan,
K.Marapakala,
A.A.Ajees,
P.Kandavelu,
B.P.Rosen.
A Disulfide Bond Cascade Mechanism For As(III) S-Adenosylmethionine Methyltransferases To Be Published.
Page generated: Tue Aug 20 03:29:41 2024
Last articles
Ca in 5S4NCa in 5S4M
Ca in 5S4L
Ca in 5QJ2
Ca in 5QJ3
Ca in 5PTP
Ca in 5PB6
Ca in 5PB5
Ca in 5PB4
Ca in 5PB3