Magnesium »
PDB 4rrk-4s1l »
4ryt »
Magnesium in PDB 4ryt: Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium
Enzymatic activity of Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium
All present enzymatic activity of Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium:
3.1.3.5; 3.1.3.6;
3.1.3.5; 3.1.3.6;
Protein crystallography data
The structure of Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium, PDB code: 4ryt
was solved by
Y.K.Mathiharan,
M.R.N.Murthy,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.00 / 2.09 |
| Space group | F 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 73.010, 121.180, 143.770, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.3 / 24.4 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium
(pdb code 4ryt). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium, PDB code: 4ryt:
In total only one binding site of Magnesium was determined in the Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium, PDB code: 4ryt:
Magnesium binding site 1 out of 1 in 4ryt
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium
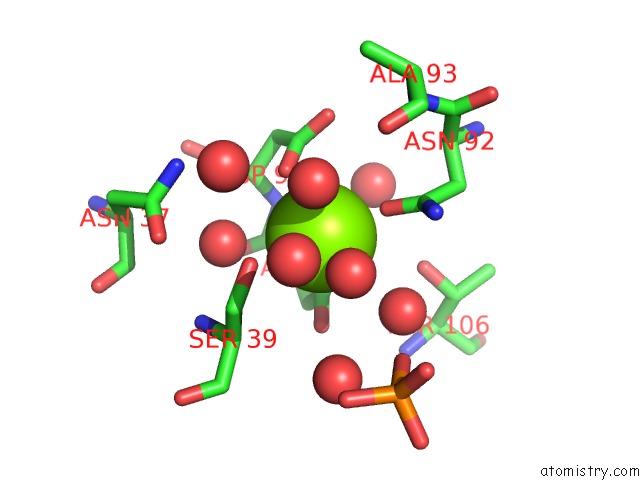
Mono view
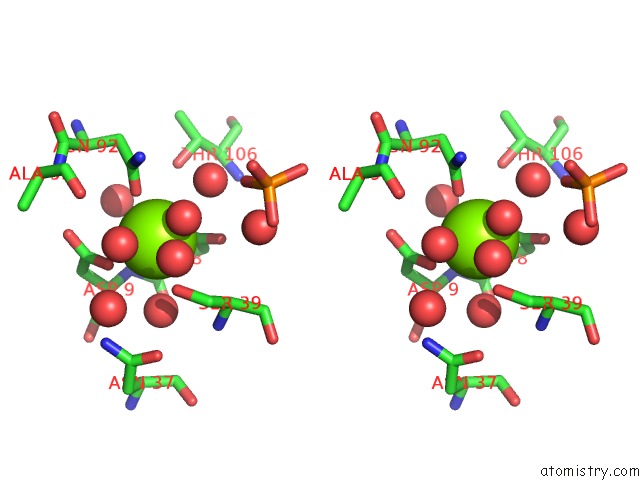
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of F222 Form of E112A Mutant of Stationary Phase Survival Protein (Sure) From Salmonella Typhimurium within 5.0Å range:
|
Reference:
Y.K.Mathiharan,
H.S.Savithri,
M.R.Murthy.
Insights Into Stabilizing Interactions in the Distorted Domain-Swapped Dimer of Salmonella Typhimurium Survival Protein. Acta Crystallogr. D Biol. V. 71 1812 2015CRYSTALLOGR..
ISSN: ESSN 1399-0047
PubMed: 26327371
DOI: 10.1107/S1399004715011992
Page generated: Tue Aug 20 03:35:49 2024
ISSN: ESSN 1399-0047
PubMed: 26327371
DOI: 10.1107/S1399004715011992
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO