Magnesium »
PDB 4xgr-4xtj »
4xj3 »
Magnesium in PDB 4xj3: Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form
Protein crystallography data
The structure of Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form, PDB code: 4xj3
was solved by
K.Kato,
R.Ishii,
R.Ishitani,
O.Nureki,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.95 / 1.65 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 118.780, 49.850, 72.860, 90.00, 93.48, 90.00 |
| R / Rfree (%) | 17.6 / 20.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form
(pdb code 4xj3). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form, PDB code: 4xj3:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form, PDB code: 4xj3:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4xj3
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form
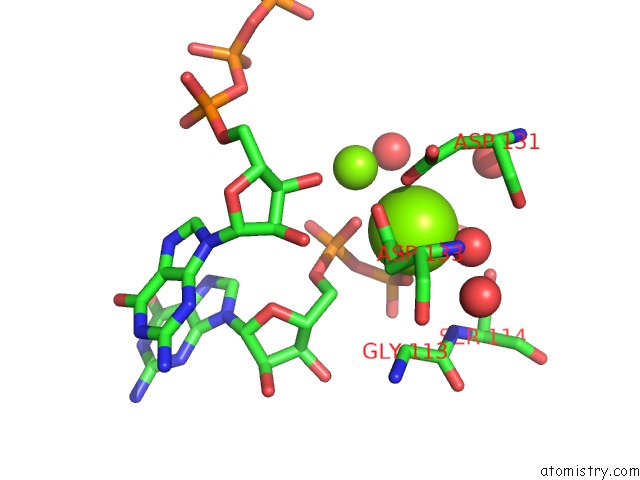
Mono view
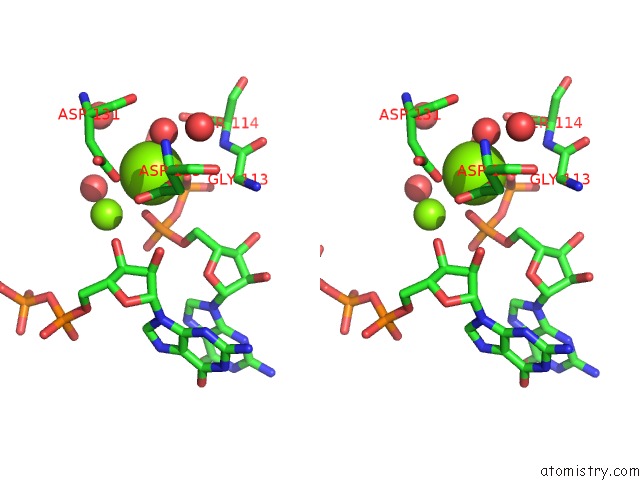
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4xj3
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form
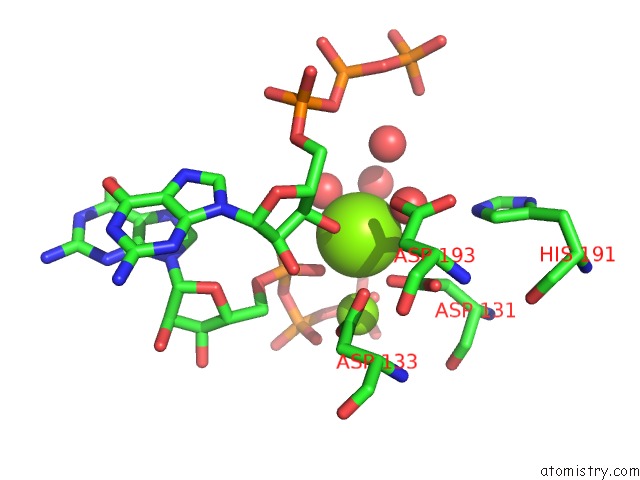
Mono view
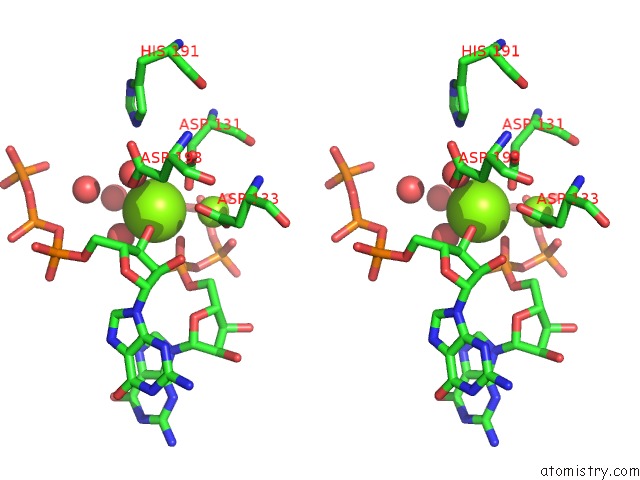
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Vibrio Cholerae Dncv Gtp Bound Form within 5.0Å range:
|
Reference:
K.Kato,
R.Ishii,
S.Hirano,
R.Ishitani,
O.Nureki.
Structural Basis For the Catalytic Mechanism of Dncv, Bacterial Homolog of Cyclic Gmp-Amp Synthase Structure V. 23 843 2015.
ISSN: ISSN 0969-2126
PubMed: 25865248
DOI: 10.1016/J.STR.2015.01.023
Page generated: Tue Aug 12 03:07:14 2025
ISSN: ISSN 0969-2126
PubMed: 25865248
DOI: 10.1016/J.STR.2015.01.023
Last articles
Mg in 5G0RMg in 5G5V
Mg in 5G3T
Mg in 5G5T
Mg in 5G5S
Mg in 5G4A
Mg in 5G57
Mg in 5G50
Mg in 5G41
Mg in 5G3Z