Magnesium »
PDB 5chi-5cr0 »
5cmc »
Magnesium in PDB 5cmc: Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex
Protein crystallography data
The structure of Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex, PDB code: 5cmc
was solved by
M.L.Mayer,
A.Thomas,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.11 / 1.28 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 45.899, 123.138, 54.507, 90.00, 111.72, 90.00 |
| R / Rfree (%) | 13.4 / 15.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex
(pdb code 5cmc). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex, PDB code: 5cmc:
In total only one binding site of Magnesium was determined in the Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex, PDB code: 5cmc:
Magnesium binding site 1 out of 1 in 5cmc
Go back to
Magnesium binding site 1 out
of 1 in the Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex
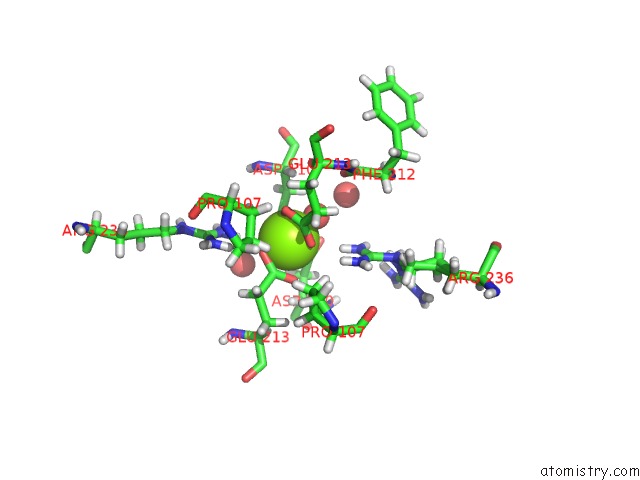
Mono view
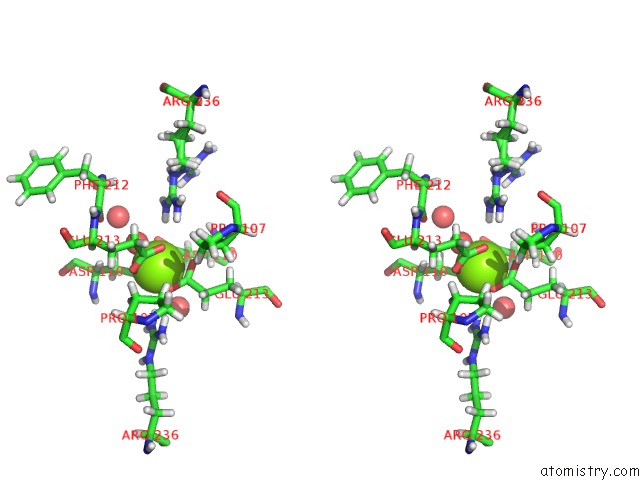
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Mnemiopsis Leidyi ML032222A Iglur Lbd E423S Mutant Glycine Complex within 5.0Å range:
|
Reference:
A.Yu,
R.Alberstein,
A.Thomas,
A.Zimmet,
R.Grey,
M.L.Mayer,
A.Y.Lau.
Molecular Lock Regulates Binding of Glycine to A Primitive Nmda Receptor. Proc.Natl.Acad.Sci.Usa V. 113 E6786 2016.
ISSN: ESSN 1091-6490
PubMed: 27791085
DOI: 10.1073/PNAS.1607010113
Page generated: Tue Aug 12 06:30:18 2025
ISSN: ESSN 1091-6490
PubMed: 27791085
DOI: 10.1073/PNAS.1607010113
Last articles
Mg in 6K27Mg in 6K7K
Mg in 6K7J
Mg in 6K77
Mg in 6K74
Mg in 6K55
Mg in 6K5P
Mg in 6K4E
Mg in 6K4Y
Mg in 6K32