Magnesium »
PDB 5dji-5ds5 »
5dmz »
Magnesium in PDB 5dmz: Structure of Human BUB1 Kinase Domain Phosphorylated at SER969
Enzymatic activity of Structure of Human BUB1 Kinase Domain Phosphorylated at SER969
All present enzymatic activity of Structure of Human BUB1 Kinase Domain Phosphorylated at SER969:
2.7.11.1;
2.7.11.1;
Protein crystallography data
The structure of Structure of Human BUB1 Kinase Domain Phosphorylated at SER969, PDB code: 5dmz
was solved by
C.Breit,
J.R.Weir,
A.Musacchio,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.30 / 2.40 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.380, 66.340, 106.540, 90.00, 98.34, 90.00 |
| R / Rfree (%) | 21.1 / 24.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Human BUB1 Kinase Domain Phosphorylated at SER969
(pdb code 5dmz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of Human BUB1 Kinase Domain Phosphorylated at SER969, PDB code: 5dmz:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of Human BUB1 Kinase Domain Phosphorylated at SER969, PDB code: 5dmz:
Jump to Magnesium binding site number: 1; 2;
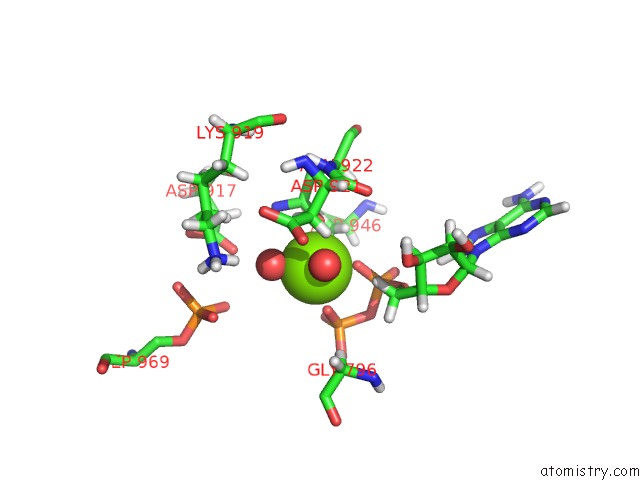
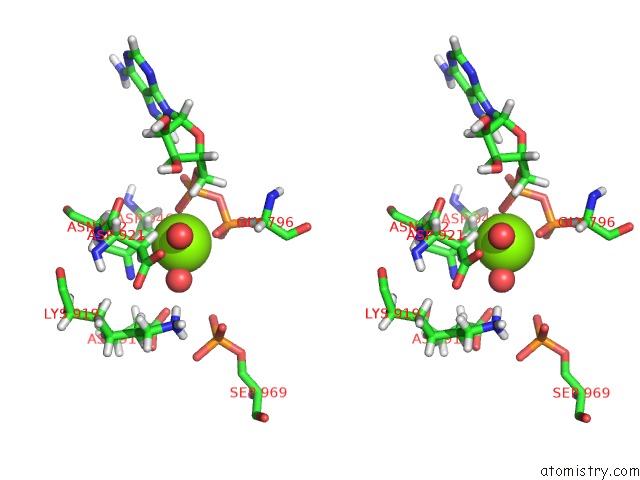
Magnesium binding site 1 out of 2 in 5dmz
Go back to
Magnesium binding site 1 out
of 2 in the Structure of Human BUB1 Kinase Domain Phosphorylated at SER969
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Human BUB1 Kinase Domain Phosphorylated at SER969 within 5.0Å range:
|
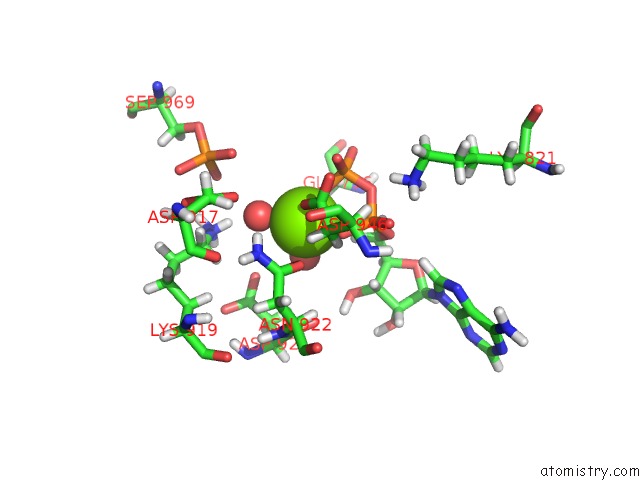
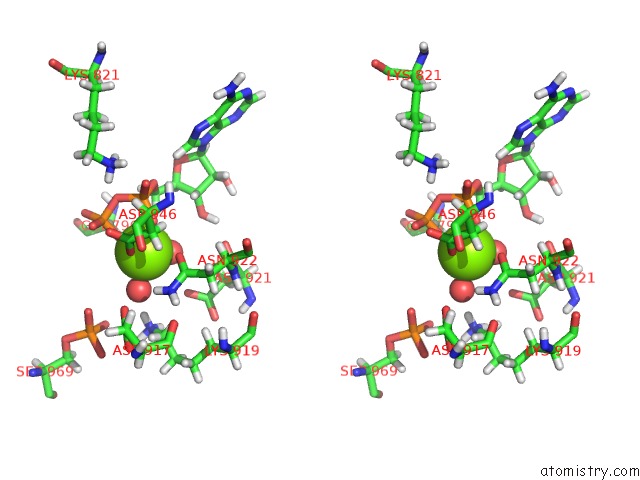
Magnesium binding site 2 out of 2 in 5dmz
Go back to
Magnesium binding site 2 out
of 2 in the Structure of Human BUB1 Kinase Domain Phosphorylated at SER969
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Human BUB1 Kinase Domain Phosphorylated at SER969 within 5.0Å range:
|
Reference:
C.Breit,
T.Bange,
A.Petrovic,
J.R.Weir,
F.Muller,
D.Vogt,
A.Musacchio.
Role of Intrinsic and Extrinsic Factors in the Regulation of the Mitotic Checkpoint Kinase BUB1. Plos One V. 10 44673 2015.
ISSN: ESSN 1932-6203
PubMed: 26658523
DOI: 10.1371/JOURNAL.PONE.0144673
Page generated: Tue Aug 12 07:02:48 2025
ISSN: ESSN 1932-6203
PubMed: 26658523
DOI: 10.1371/JOURNAL.PONE.0144673
Last articles
Mg in 5HKVMg in 5HR6
Mg in 5HQL
Mg in 5HQW
Mg in 5HQM
Mg in 5HQC
Mg in 5HQB
Mg in 5HQ8
Mg in 5HQA
Mg in 5HQ4