Magnesium »
PDB 5e7c-5egc »
5edm »
Magnesium in PDB 5edm: Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
Enzymatic activity of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
All present enzymatic activity of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ):
3.4.21.5;
3.4.21.5;
Protein crystallography data
The structure of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ), PDB code: 5edm
was solved by
N.Pozzi,
Z.Chen,
E.Di Cera,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 92.07 / 2.20 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 109.884, 168.690, 144.315, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.6 / 23.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
(pdb code 5edm). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ), PDB code: 5edm:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ), PDB code: 5edm:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 5edm
Go back to
Magnesium binding site 1 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
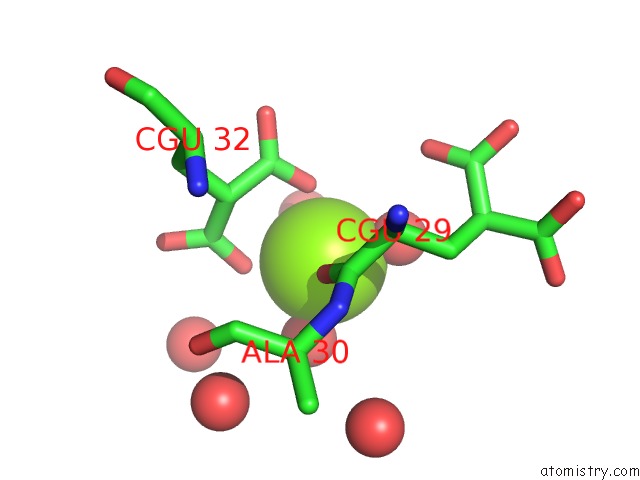
Mono view
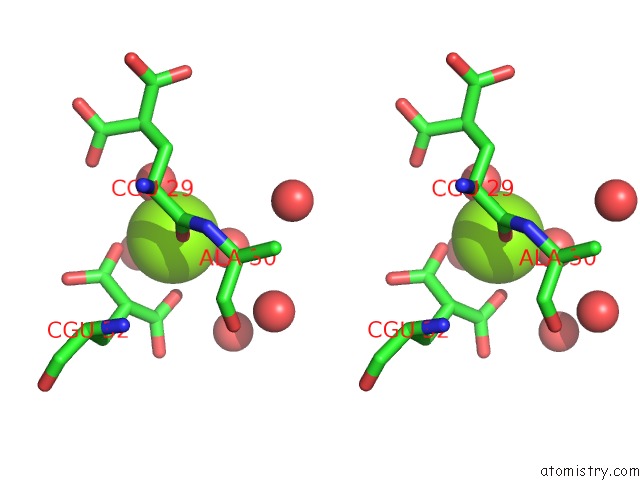
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 5edm
Go back to
Magnesium binding site 2 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
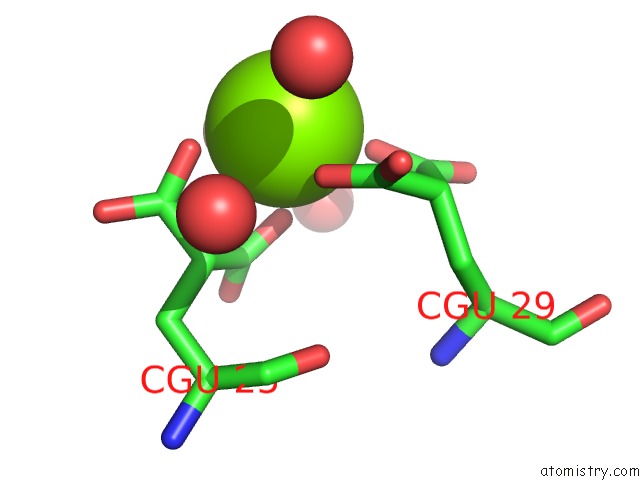
Mono view
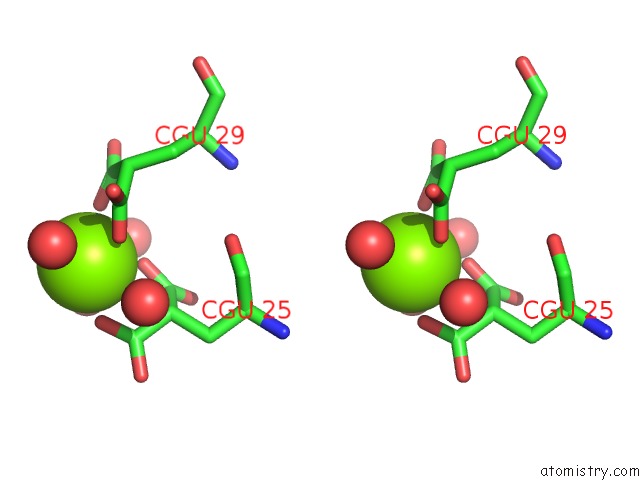
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 5edm
Go back to
Magnesium binding site 3 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
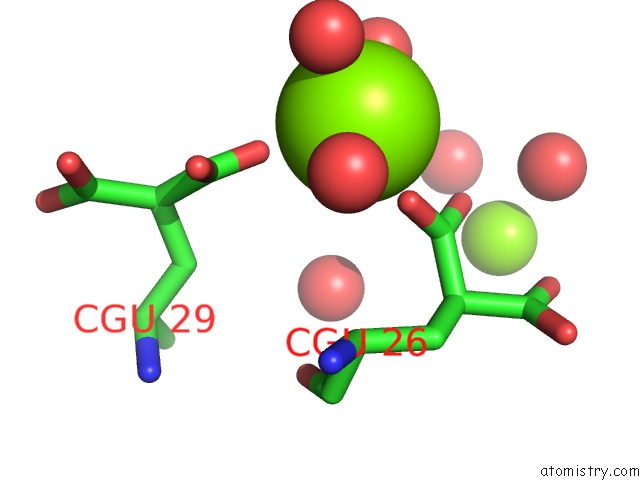
Mono view
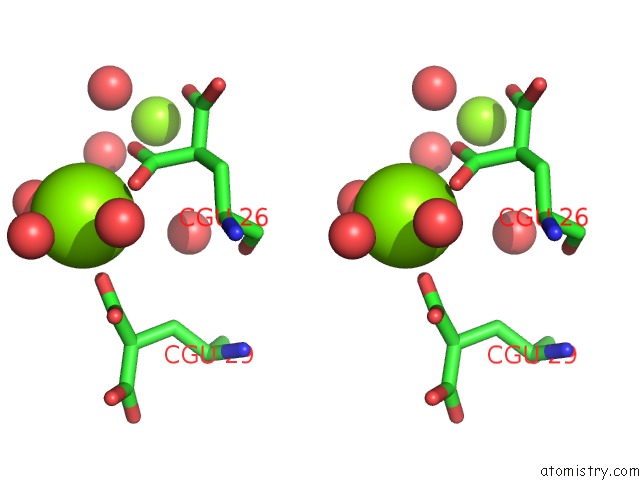
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 5edm
Go back to
Magnesium binding site 4 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
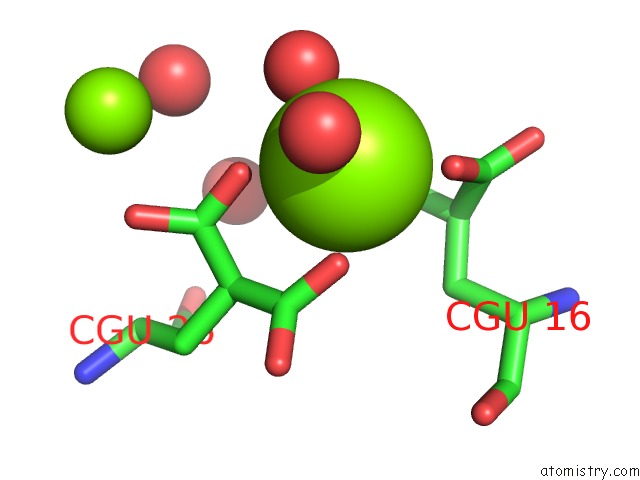
Mono view
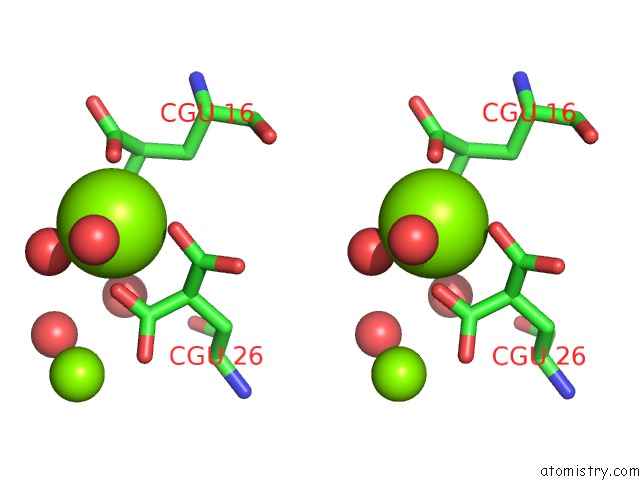
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 5edm
Go back to
Magnesium binding site 5 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
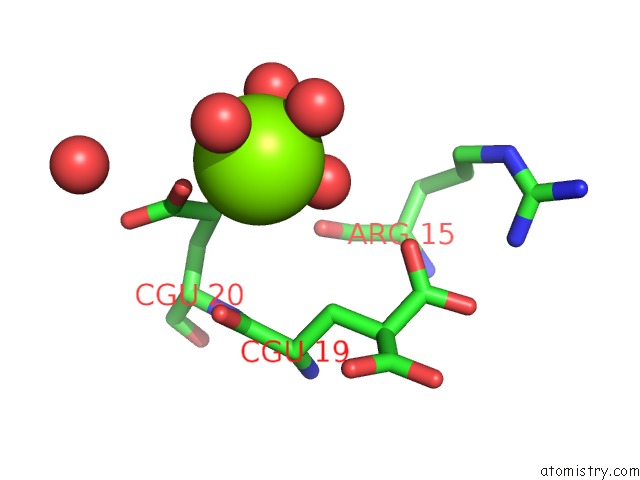
Mono view
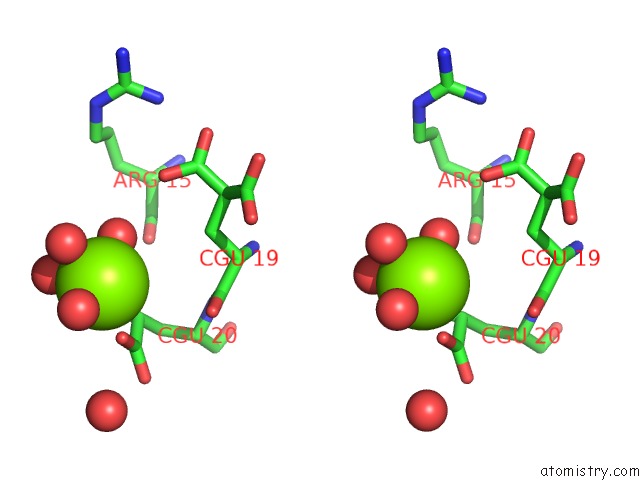
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 5edm
Go back to
Magnesium binding site 6 out
of 6 in the Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I )
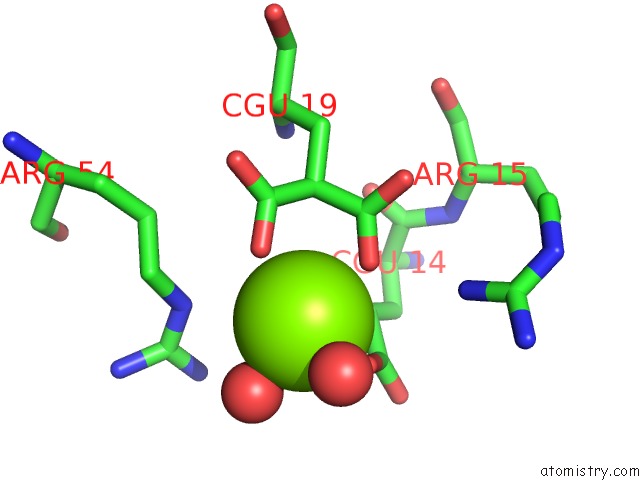
Mono view
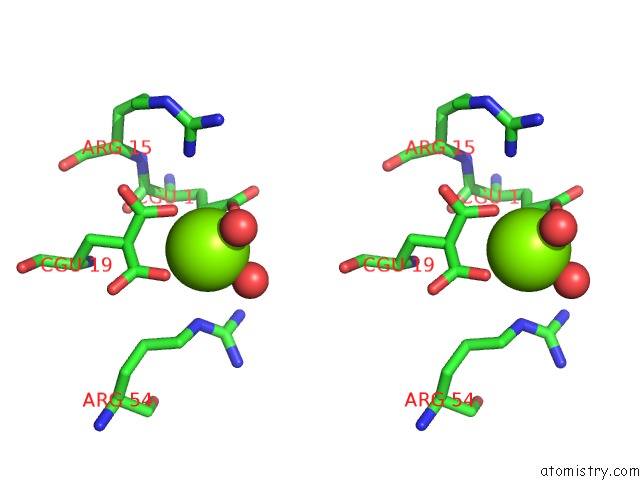
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Prothrombin Deletion Mutant Residues 154-167 ( Form I ) within 5.0Å range:
|
Reference:
N.Pozzi,
Z.Chen,
E.Di Cera.
How the Linker Connecting the Two Kringles Influences Activation and Conformational Plasticity of Prothrombin. J.Biol.Chem. V. 291 6071 2016.
ISSN: ESSN 1083-351X
PubMed: 26763231
DOI: 10.1074/JBC.M115.700401
Page generated: Sun Sep 29 03:38:36 2024
ISSN: ESSN 1083-351X
PubMed: 26763231
DOI: 10.1074/JBC.M115.700401
Last articles
Cl in 5XIOCl in 5XHI
Cl in 5XIH
Cl in 5XHO
Cl in 5XIF
Cl in 5XHN
Cl in 5XHM
Cl in 5XGP
Cl in 5XH7
Cl in 5XH6