Magnesium »
PDB 5egy-5etr »
5eq9 »
Magnesium in PDB 5eq9: Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
Protein crystallography data
The structure of Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+, PDB code: 5eq9
was solved by
M.Ruszkowski,
Z.Dauter,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.25 / 1.36 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 61.885, 89.802, 92.593, 90.00, 97.07, 90.00 |
| R / Rfree (%) | 11.6 / 15.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
(pdb code 5eq9). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+, PDB code: 5eq9:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+, PDB code: 5eq9:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 5eq9
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
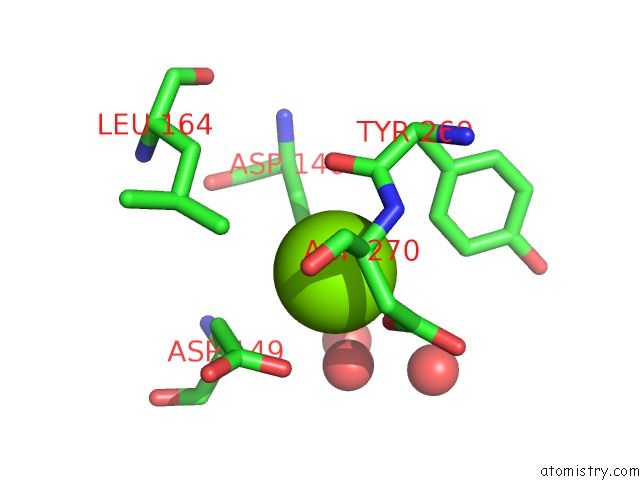
Mono view
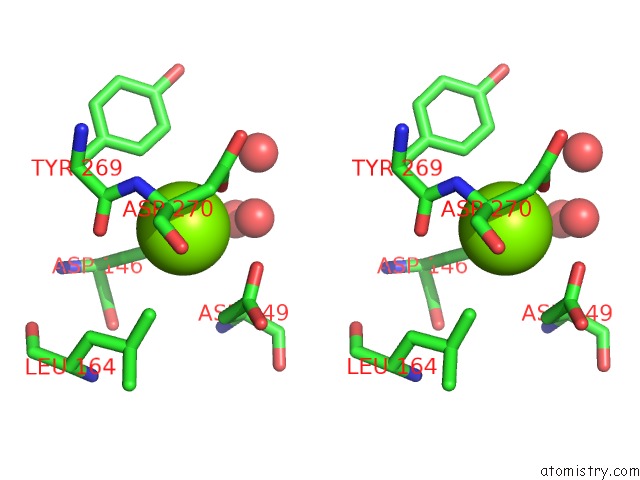
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+ within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 5eq9
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
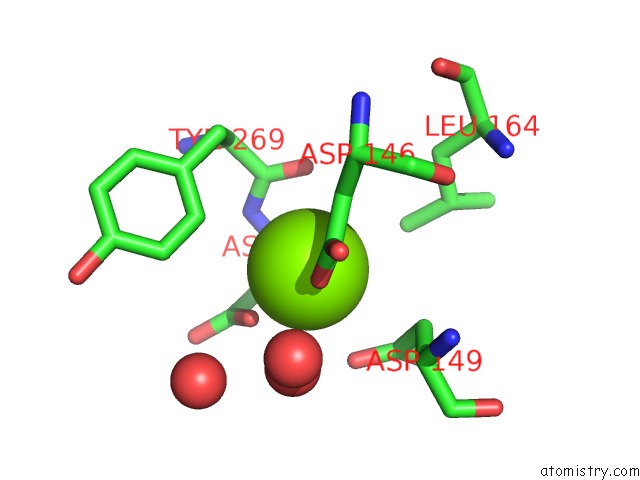
Mono view
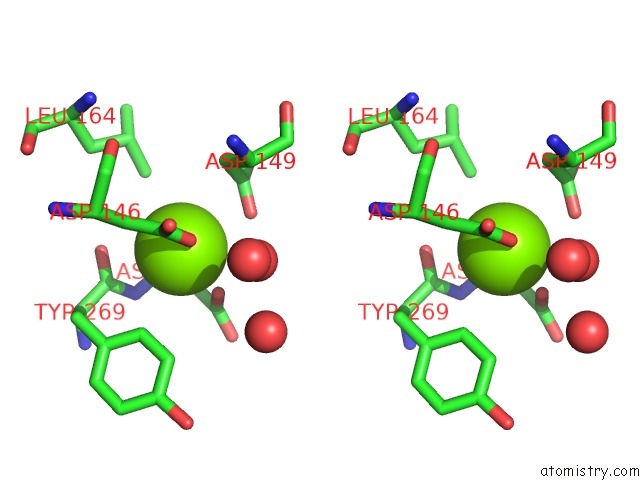
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+ within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 5eq9
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
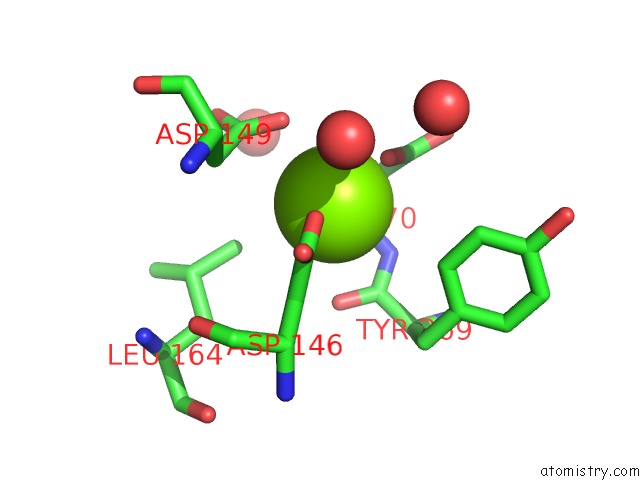
Mono view
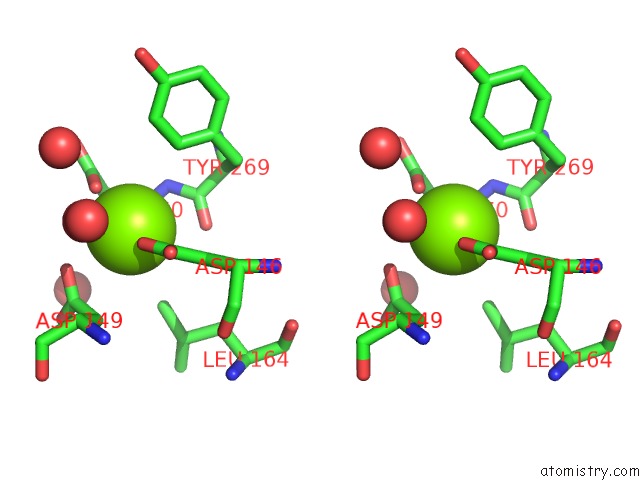
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+ within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 5eq9
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+
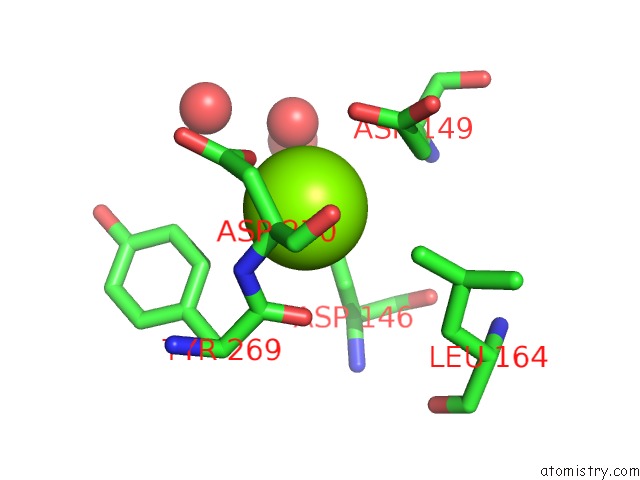
Mono view
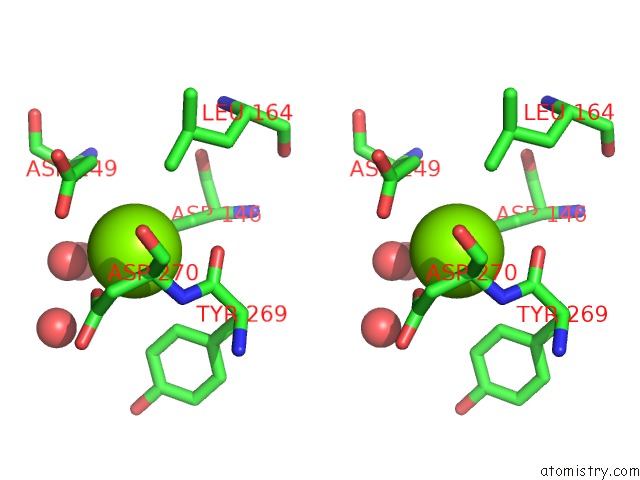
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Medicago Truncatula Histidinol-Phosphate Phosphatase (Mthpp) in Complex with L-Histidinol Phosphate and MG2+ within 5.0Å range:
|
Reference:
M.Ruszkowski,
Z.Dauter.
Structural Studies of Medicago Truncatula Histidinol Phosphate Phosphatase From Inositol Monophosphatase Superfamily Reveal Details of Penultimate Step of Histidine Biosynthesis in Plants. J.Biol.Chem. V. 291 9960 2016.
ISSN: ESSN 1083-351X
PubMed: 26994138
DOI: 10.1074/JBC.M115.708727
Page generated: Sun Sep 29 03:53:23 2024
ISSN: ESSN 1083-351X
PubMed: 26994138
DOI: 10.1074/JBC.M115.708727
Last articles
Cl in 5YT7Cl in 5YSU
Cl in 5YQH
Cl in 5YRO
Cl in 5YKY
Cl in 5YQA
Cl in 5YPL
Cl in 5YPK
Cl in 5YPJ
Cl in 5YPI