Magnesium »
PDB 5egc-5etq »
5ess »
Magnesium in PDB 5ess: Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
Enzymatic activity of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
All present enzymatic activity of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct):
2.2.1.9;
2.2.1.9;
Protein crystallography data
The structure of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct), PDB code: 5ess
was solved by
J.M.Johnston,
E.N.M.Jirgis,
G.Bashiri,
E.M.M.Bulloch,
E.N.Baker,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.73 / 2.20 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 101.144, 143.551, 174.289, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.6 / 22.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
(pdb code 5ess). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct), PDB code: 5ess:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct), PDB code: 5ess:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 5ess
Go back to
Magnesium binding site 1 out
of 3 in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
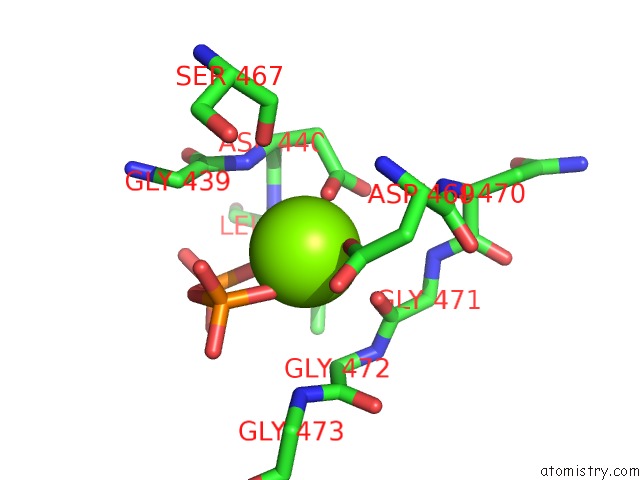
Mono view
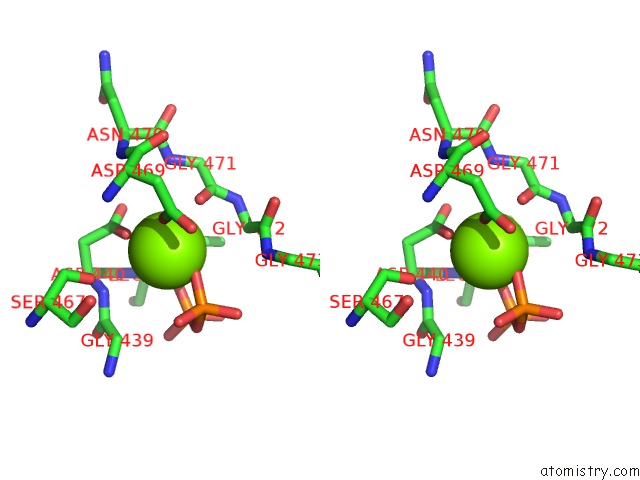
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct) within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 5ess
Go back to
Magnesium binding site 2 out
of 3 in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
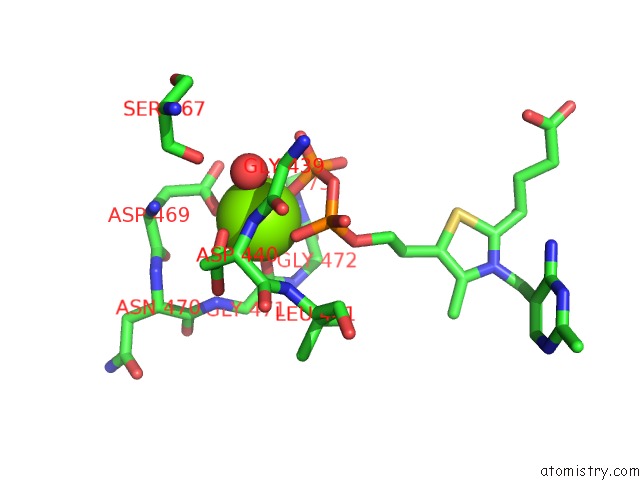
Mono view
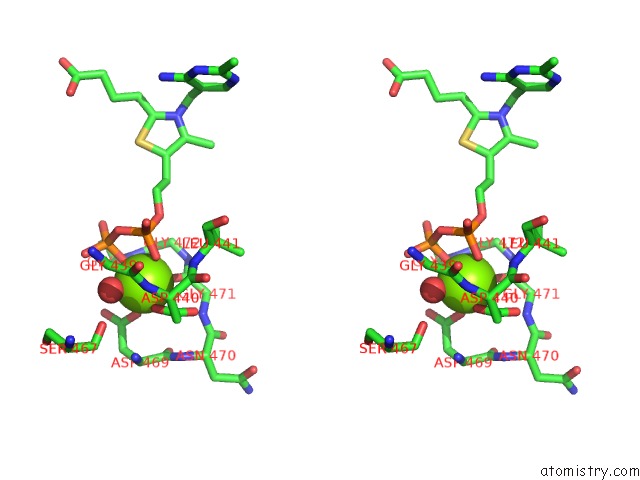
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct) within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 5ess
Go back to
Magnesium binding site 3 out
of 3 in the Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct)
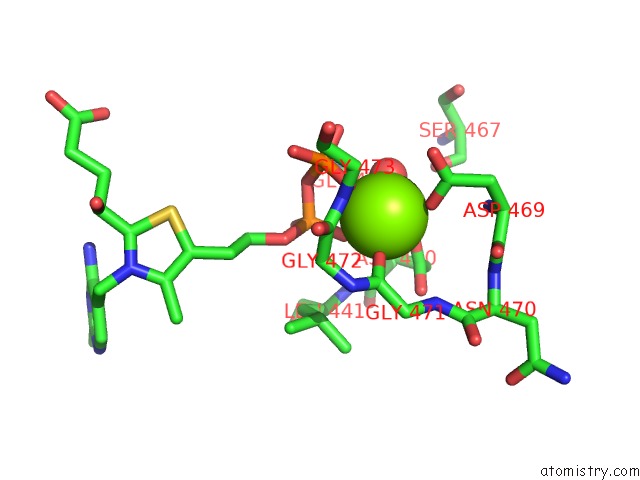
Mono view
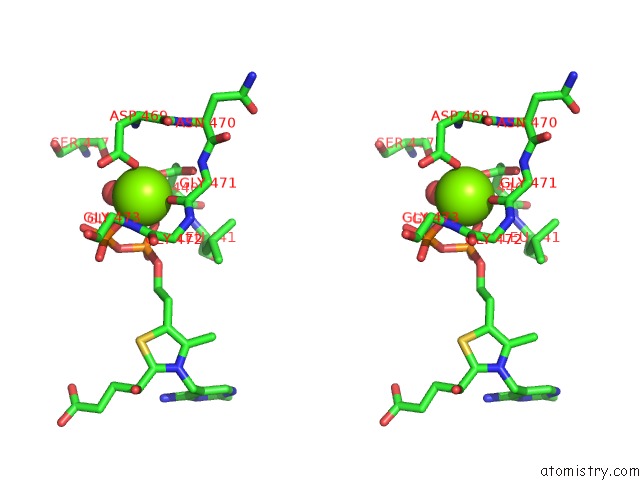
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of M. Tuberculosis Mend Bound to MG2+ and Covalent Intermediate I (A Thdp and Decarboxylated 2-Oxoglutarate Adduct) within 5.0Å range:
|
Reference:
E.N.Jirgis,
G.Bashiri,
E.M.Bulloch,
J.M.Johnston,
E.N.Baker.
Structural Views Along the Mycobacterium Tuberculosis Mend Reaction Pathway Illuminate Key Aspects of Thiamin Diphosphate-Dependent Enzyme Mechanisms. Structure V. 24 1167 2016.
ISSN: ISSN 0969-2126
PubMed: 27291649
DOI: 10.1016/J.STR.2016.04.018
Page generated: Sun Sep 29 03:54:51 2024
ISSN: ISSN 0969-2126
PubMed: 27291649
DOI: 10.1016/J.STR.2016.04.018
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW