Magnesium »
PDB 5grf-5h2f »
5grf »
Magnesium in PDB 5grf: Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp
Enzymatic activity of Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp
All present enzymatic activity of Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp:
1.1.1.41;
1.1.1.41;
Protein crystallography data
The structure of Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp, PDB code: 5grf
was solved by
T.Ma,
J.Ding,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.51 / 2.50 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.336, 114.336, 143.901, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.7 / 24.8 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp
(pdb code 5grf). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp, PDB code: 5grf:
In total only one binding site of Magnesium was determined in the Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp, PDB code: 5grf:
Magnesium binding site 1 out of 1 in 5grf
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp

Mono view
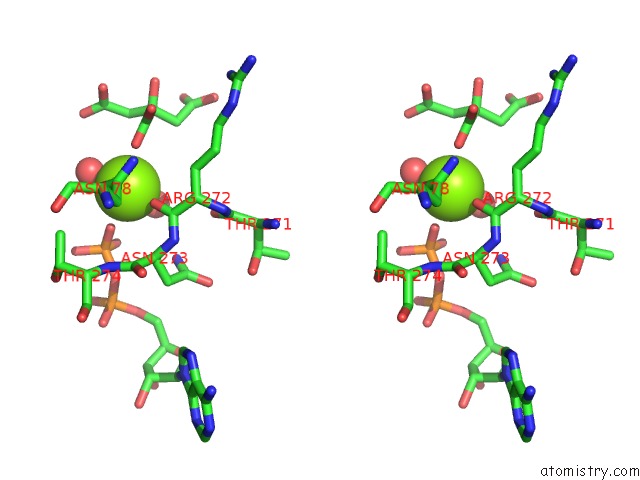
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of the Alpha Gamma Mutant (Gamma-K151A) of Human IDH3 in Complex with Mg(2+), Citrate and Adp within 5.0Å range:
|
Reference:
T.Ma,
Y.Peng,
W.Huang,
J.Ding.
Molecular Mechanism of the Allosteric Regulation of the Alpha Gamma Heterodimer of Human Nad-Dependent Isocitrate Dehydrogenase. Sci Rep V. 7 40921 2017.
ISSN: ESSN 2045-2322
PubMed: 28098230
DOI: 10.1038/SREP40921
Page generated: Sun Sep 29 15:25:25 2024
ISSN: ESSN 2045-2322
PubMed: 28098230
DOI: 10.1038/SREP40921
Last articles
F in 7JK8F in 7JHW
F in 7JHD
F in 7I18
F in 7I2F
F in 7I2M
F in 7I2A
F in 7I2D
F in 7HNS
F in 7HOG