Magnesium »
PDB 5ld1-5lmn »
5li1 »
Magnesium in PDB 5li1: Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain
Enzymatic activity of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain
All present enzymatic activity of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain:
2.7.11.13;
2.7.11.13;
Protein crystallography data
The structure of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain, PDB code: 5li1
was solved by
E.V.Soriano,
A.G.Purkiss,
N.Q.Mcdonald,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.01 / 2.00 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.030, 82.030, 90.790, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 15 / 21.7 |
Other elements in 5li1:
The structure of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain also contains other interesting chemical elements:
| Potassium | (K) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain
(pdb code 5li1). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain, PDB code: 5li1:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain, PDB code: 5li1:
Jump to Magnesium binding site number: 1; 2;
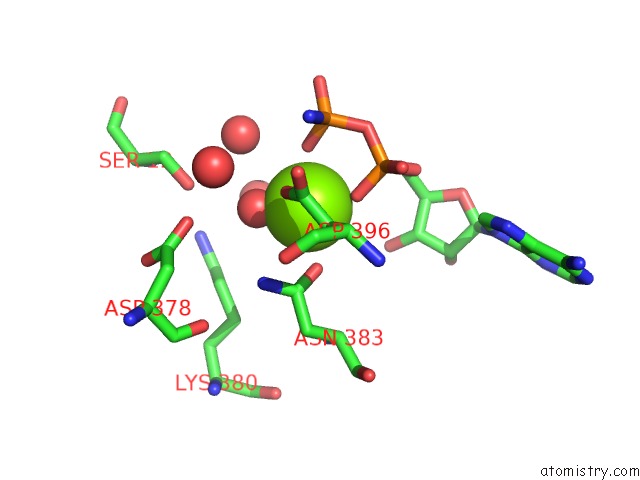
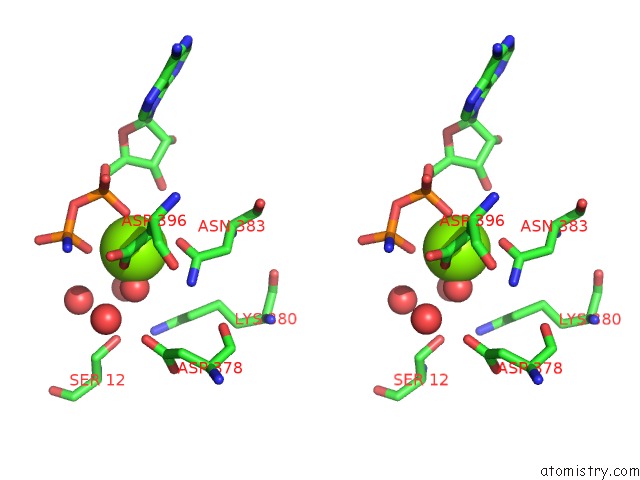
Magnesium binding site 1 out of 2 in 5li1
Go back to
Magnesium binding site 1 out
of 2 in the Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain within 5.0Å range:
|
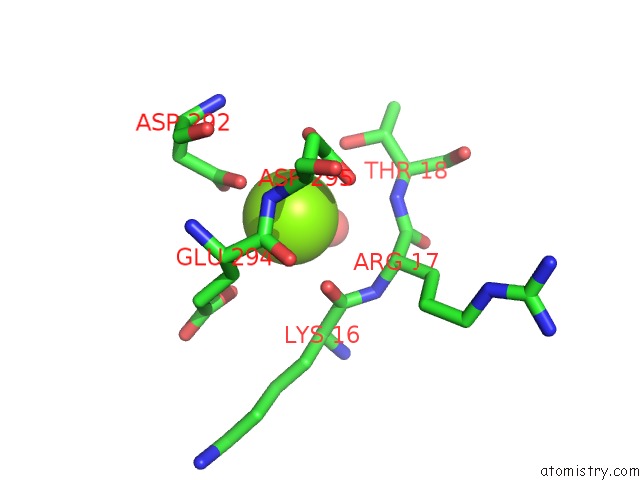
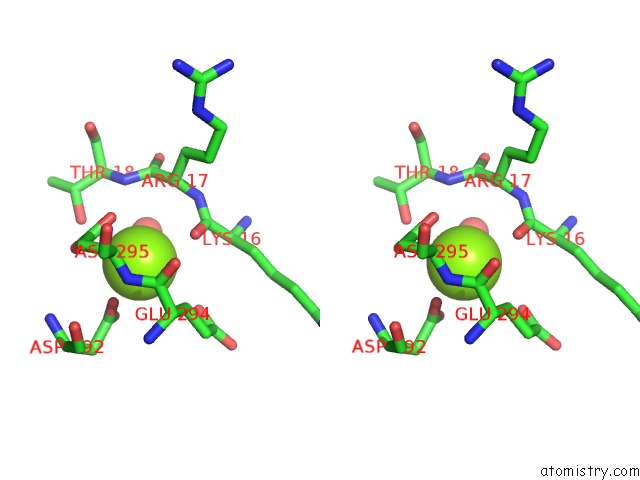
Magnesium binding site 2 out of 2 in 5li1
Go back to
Magnesium binding site 2 out
of 2 in the Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of A PAR3-Inhibitory Peptide Bound to Pkciota Core Kinase Domain within 5.0Å range:
|
Reference:
E.V.Soriano,
M.E.Ivanova,
G.Fletcher,
P.Riou,
P.P.Knowles,
K.Barnouin,
A.Purkiss,
B.Kostelecky,
P.Saiu,
M.Linch,
A.Elbediwy,
S.Kjr,
N.O'reilly,
A.P.Snijders,
P.J.Parker,
B.J.Thompson,
N.Q.Mcdonald.
Apkc Inhibition By PAR3 CR3 Flanking Regions Controls Substrate Access and Underpins Apical-Junctional Polarization. Dev.Cell V. 38 384 2016.
ISSN: ISSN 1534-5807
PubMed: 27554858
DOI: 10.1016/J.DEVCEL.2016.07.018
Page generated: Sun Sep 29 20:12:25 2024
ISSN: ISSN 1534-5807
PubMed: 27554858
DOI: 10.1016/J.DEVCEL.2016.07.018
Last articles
Ca in 5SWICa in 5SVE
Ca in 5SSX
Ca in 5SV0
Ca in 5STD
Ca in 5SSZ
Ca in 5SSY
Ca in 5SIC
Ca in 5SBD
Ca in 5SBE