Magnesium »
PDB 5ld1-5lmn »
5llb »
Magnesium in PDB 5llb: Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
Enzymatic activity of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
All present enzymatic activity of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate:
2.7.4.1;
2.7.4.1;
Protein crystallography data
The structure of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate, PDB code: 5llb
was solved by
P.L.Roach,
A.E.Parnell,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 72.46 / 1.92 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.700, 144.910, 70.590, 90.00, 113.04, 90.00 |
| R / Rfree (%) | 17.9 / 23.1 |
Other elements in 5llb:
The structure of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate also contains other interesting chemical elements:
| Chlorine | (Cl) | 4 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
(pdb code 5llb). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate, PDB code: 5llb:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate, PDB code: 5llb:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 5llb
Go back to
Magnesium binding site 1 out
of 4 in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
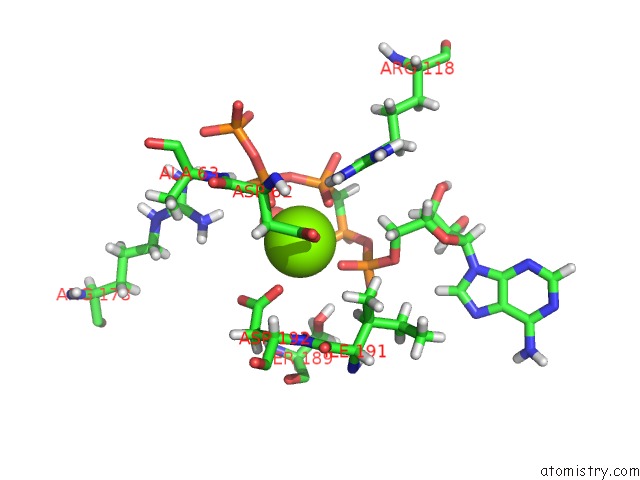
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate within 5.0Å range:
|
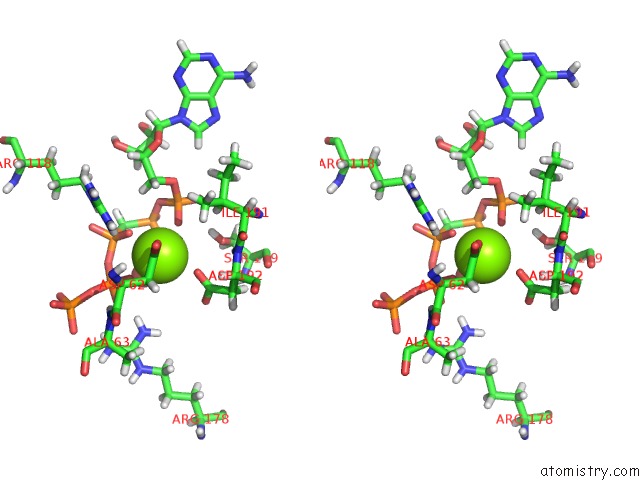
Magnesium binding site 2 out of 4 in 5llb
Go back to
Magnesium binding site 2 out
of 4 in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate within 5.0Å range:
|
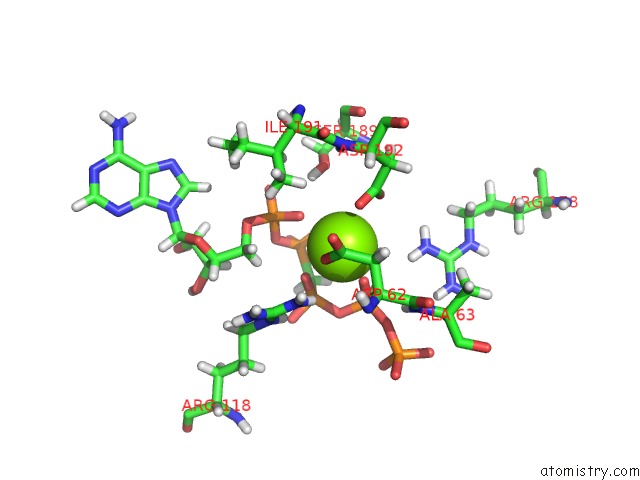
Magnesium binding site 3 out of 4 in 5llb
Go back to
Magnesium binding site 3 out
of 4 in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
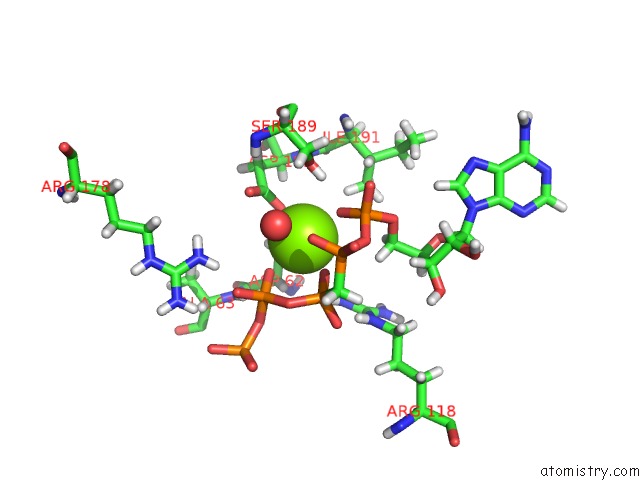
Mono view
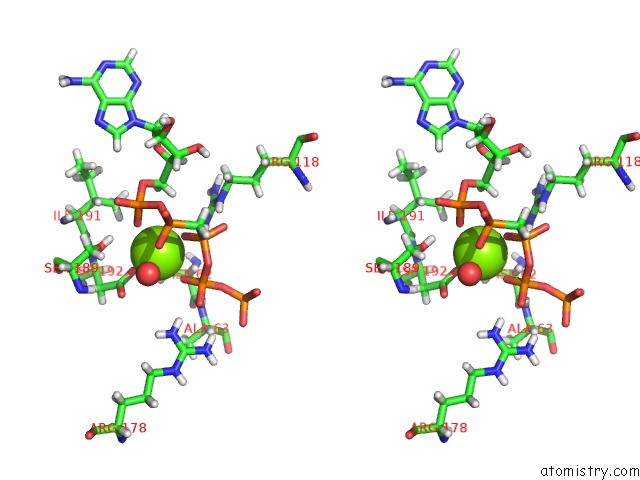
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate within 5.0Å range:
|
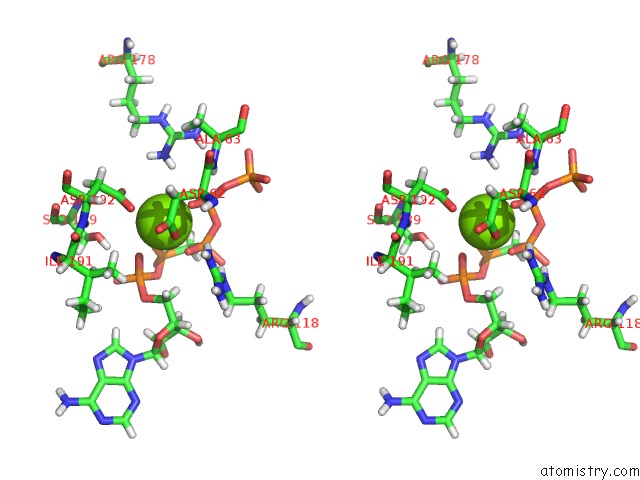
Magnesium binding site 4 out of 4 in 5llb
Go back to
Magnesium binding site 4 out
of 4 in the Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate
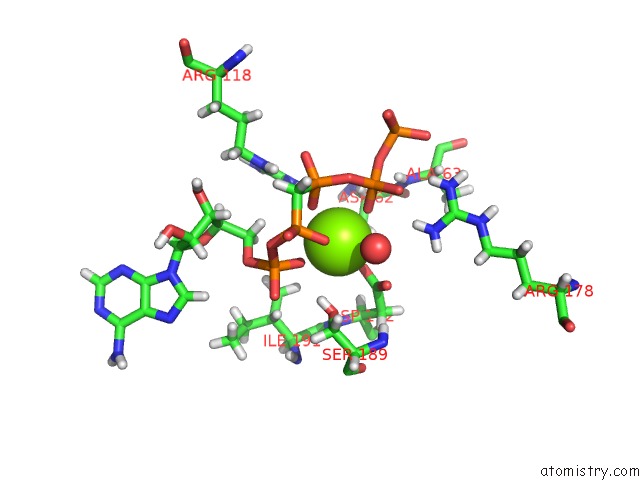
Mono view
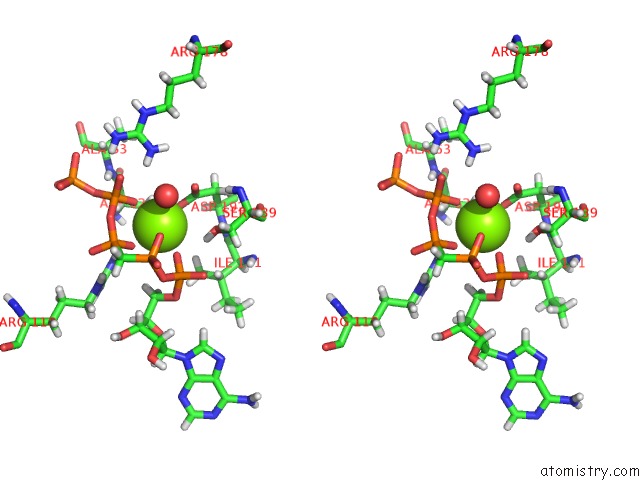
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Polyphosphate Kinase 2 From Francisella Tularensis with AMPPCH2PPP and Polyphosphate within 5.0Å range:
|
Reference:
A.E.Parnell,
S.Mordhorst,
F.Kemper,
M.Giurrandino,
J.P.Prince,
N.J.Schwarzer,
A.Hofer,
D.Wohlwend,
H.J.Jessen,
S.Gerhardt,
O.Einsle,
P.C.F.Oyston,
J.N.Andexer,
P.L.Roach.
Substrate Recognition and Mechanism Revealed By Ligand-Bound Polyphosphate Kinase 2 Structures. Proc. Natl. Acad. Sci. V. 115 3350 2018U.S.A..
ISSN: ESSN 1091-6490
PubMed: 29531036
DOI: 10.1073/PNAS.1710741115
Page generated: Sun Sep 29 20:15:58 2024
ISSN: ESSN 1091-6490
PubMed: 29531036
DOI: 10.1073/PNAS.1710741115
Last articles
F in 4IFVF in 4IDO
F in 4ICC
F in 4IBI
F in 4IAH
F in 4IAE
F in 4I9H
F in 4I9N
F in 4I9O
F in 4IA9