Magnesium »
PDB 5mh6-5mtv »
5mha »
Magnesium in PDB 5mha: D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
Protein crystallography data
The structure of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution), PDB code: 5mha
was solved by
C.Bisson,
P.J.Baker,
J.Domenech Perez,
N.Pramanpol,
S.E.Harding,
D.W.Rice,
J.Ferrer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.15 / 1.57 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.540, 76.300, 66.990, 90.00, 97.04, 90.00 |
| R / Rfree (%) | 17.4 / 21.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
(pdb code 5mha). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 7 binding sites of Magnesium where determined in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution), PDB code: 5mha:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Magnesium where determined in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution), PDB code: 5mha:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
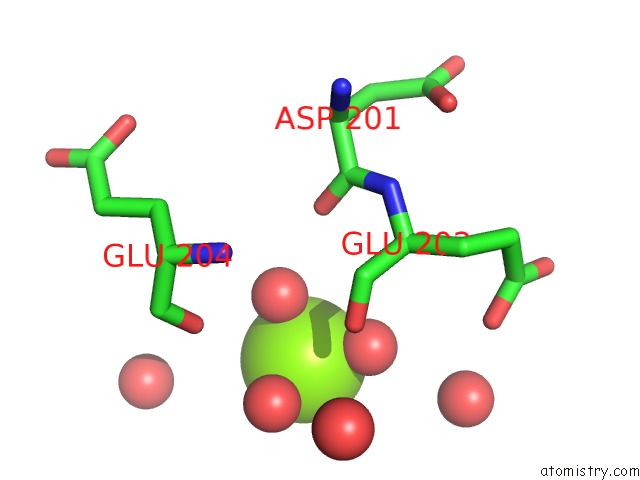
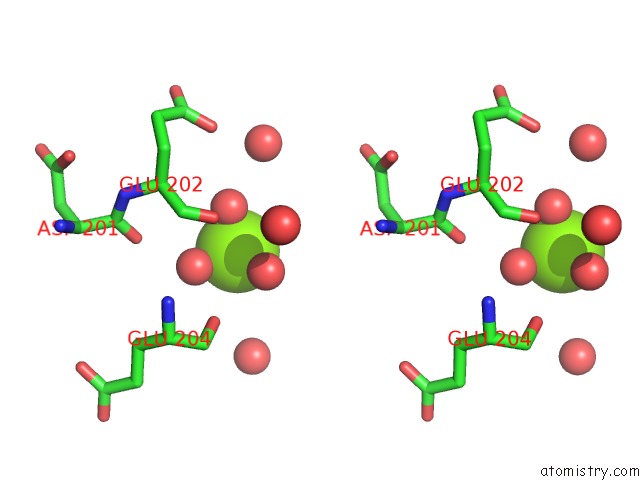
Magnesium binding site 1 out of 7 in 5mha
Go back to
Magnesium binding site 1 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
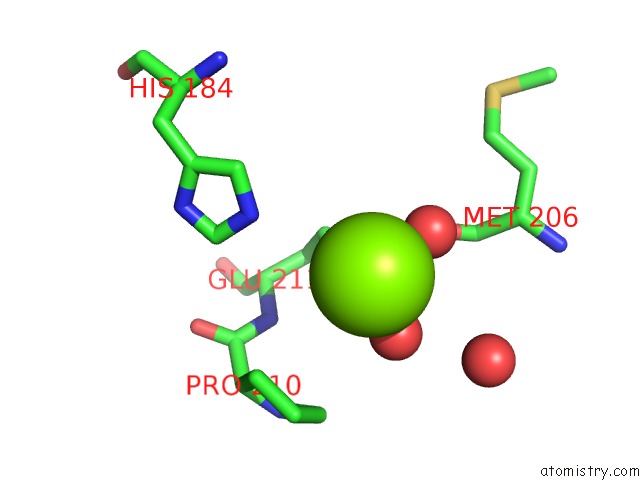
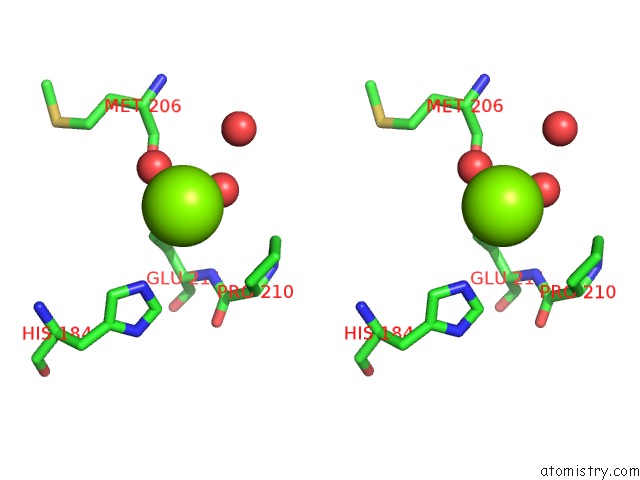
Magnesium binding site 2 out of 7 in 5mha
Go back to
Magnesium binding site 2 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Magnesium binding site 3 out of 7 in 5mha
Go back to
Magnesium binding site 3 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
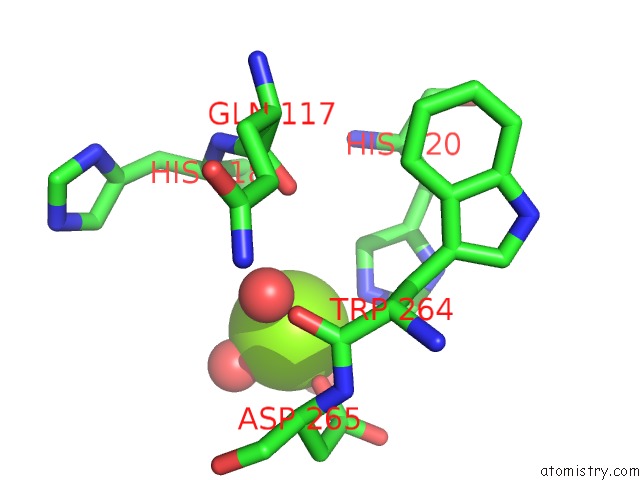
Mono view
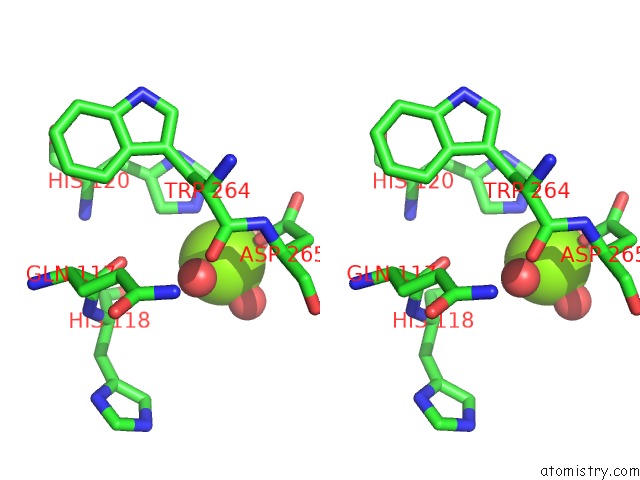
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Magnesium binding site 4 out of 7 in 5mha
Go back to
Magnesium binding site 4 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
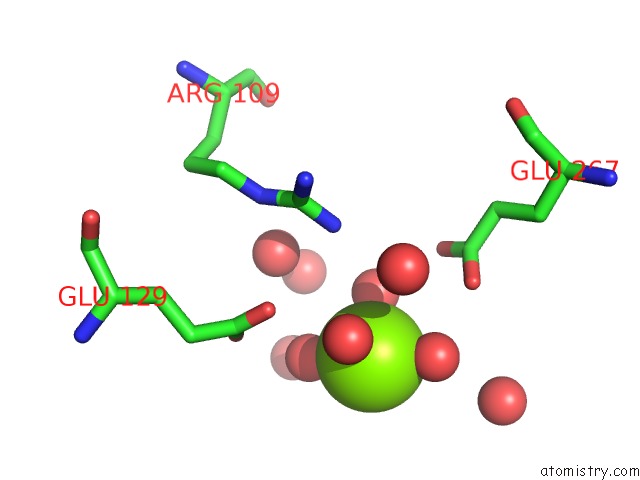
Mono view
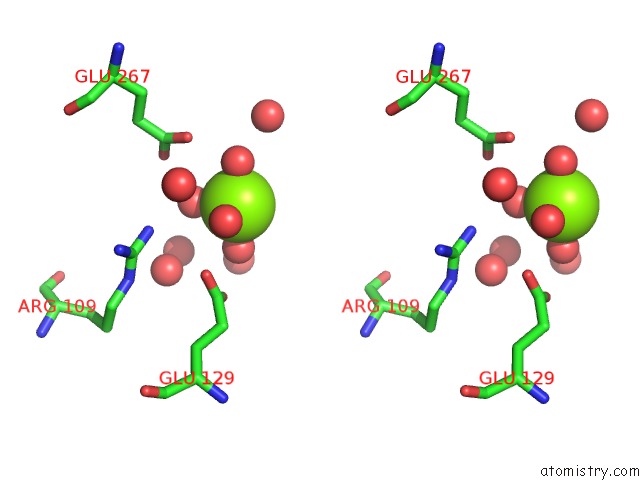
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Magnesium binding site 5 out of 7 in 5mha
Go back to
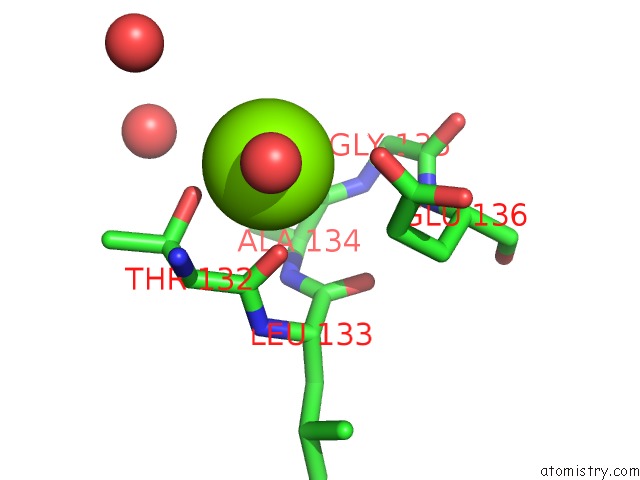
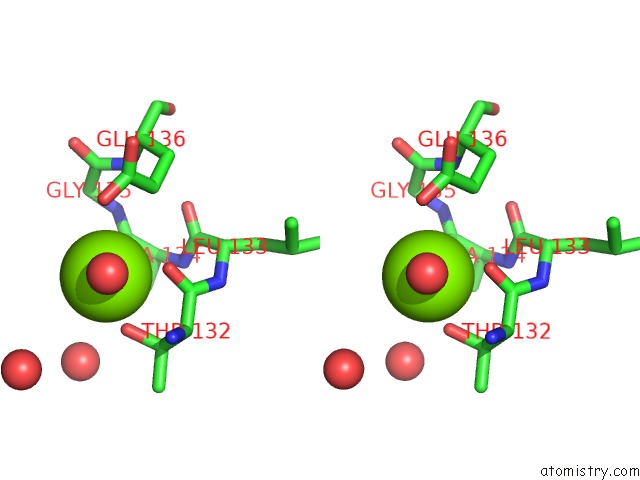
Magnesium binding site 5 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Magnesium binding site 6 out of 7 in 5mha
Go back to
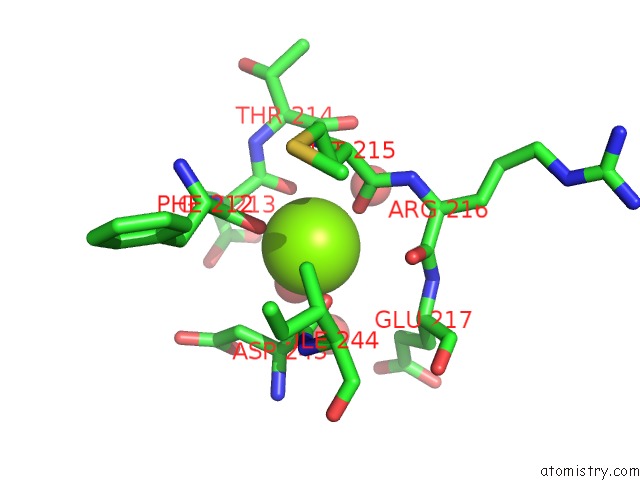
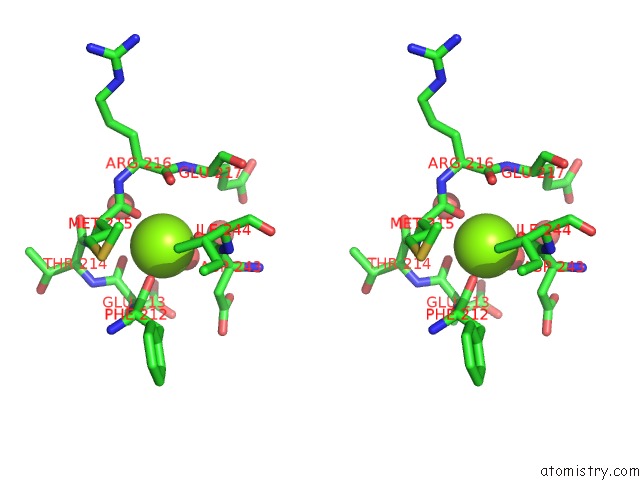
Magnesium binding site 6 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Magnesium binding site 7 out of 7 in 5mha
Go back to
Magnesium binding site 7 out
of 7 in the D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution)
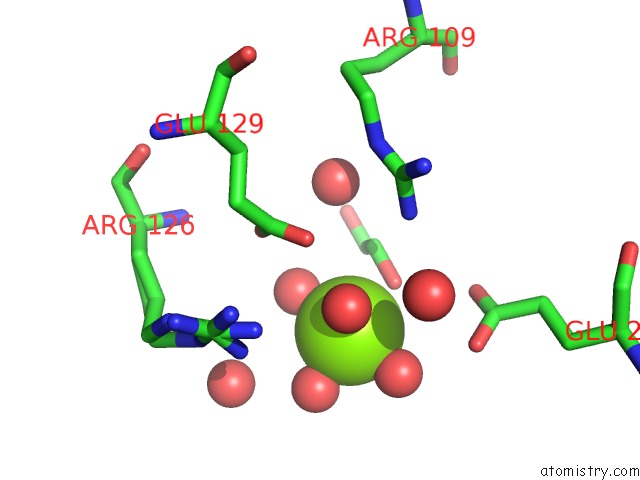
Mono view
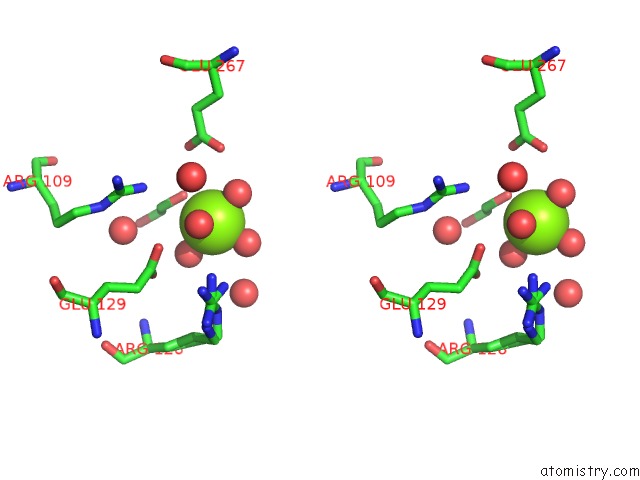
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of D-2-Hydroxyacid Dehydrogenases (D2-Hdh) From Haloferax Mediterranei in Complex with A Mixture of 2-Ketohexanoic Acid and 2-Hydroxyhexanoic Acid, and Nadph (1.57 A Resolution) within 5.0Å range:
|
Reference:
J.Domenech Perez,
N.Pramanpol,
P.J.Baker,
C.Bisson,
S.E.Harding,
D.W.Rice,
J.Ferrer.
Productive Ternary Complexes of D-2-Hydroxyacid Dehydrogenase Provide Insights Into the Chiral Specificity of Its Reaction Mechanism To Be Published.
Page generated: Sun Sep 29 21:56:13 2024
Last articles
Ca in 5TNCCa in 5TP9
Ca in 5TP0
Ca in 5TMN
Ca in 5TLI
Ca in 5TLN
Ca in 5TH6
Ca in 5TL8
Ca in 5TIW
Ca in 5TJ6