Magnesium »
PDB 5w5c-5whe »
5wg3 »
Magnesium in PDB 5wg3: Human GRK2 in Complex with Gbetagamma Subunits and CCG258748
Enzymatic activity of Human GRK2 in Complex with Gbetagamma Subunits and CCG258748
All present enzymatic activity of Human GRK2 in Complex with Gbetagamma Subunits and CCG258748:
2.7.11.15;
2.7.11.15;
Protein crystallography data
The structure of Human GRK2 in Complex with Gbetagamma Subunits and CCG258748, PDB code: 5wg3
was solved by
R.Bouley,
J.J.G.Tesmer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.07 / 2.90 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 61.267, 241.569, 214.813, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.8 / 24.8 |
Other elements in 5wg3:
The structure of Human GRK2 in Complex with Gbetagamma Subunits and CCG258748 also contains other interesting chemical elements:
| Fluorine | (F) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Human GRK2 in Complex with Gbetagamma Subunits and CCG258748
(pdb code 5wg3). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Human GRK2 in Complex with Gbetagamma Subunits and CCG258748, PDB code: 5wg3:
In total only one binding site of Magnesium was determined in the Human GRK2 in Complex with Gbetagamma Subunits and CCG258748, PDB code: 5wg3:
Magnesium binding site 1 out of 1 in 5wg3
Go back to
Magnesium binding site 1 out
of 1 in the Human GRK2 in Complex with Gbetagamma Subunits and CCG258748
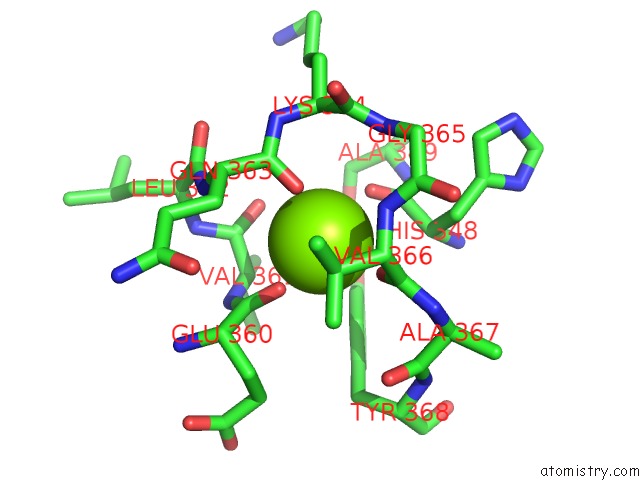
Mono view
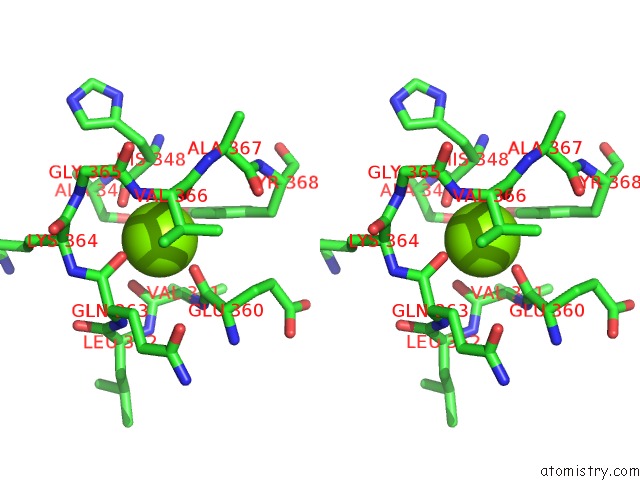
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Human GRK2 in Complex with Gbetagamma Subunits and CCG258748 within 5.0Å range:
|
Reference:
R.Bouley,
H.V.Waldschmidt,
M.C.Cato,
A.Cannavo,
J.Song,
J.Y.Cheung,
X.Q.Yao,
W.J.Koch,
S.D.Larsen,
J.J.G.Tesmer.
Structural Determinants Influencing the Potency and Selectivity of Indazole-Paroxetine Hybrid G Protein-Coupled Receptor Kinase 2 Inhibitors. Mol. Pharmacol. V. 92 707 2017.
ISSN: ESSN 1521-0111
PubMed: 29070696
DOI: 10.1124/MOL.117.110130
Page generated: Mon Sep 30 06:34:24 2024
ISSN: ESSN 1521-0111
PubMed: 29070696
DOI: 10.1124/MOL.117.110130
Last articles
Cl in 6AUKCl in 6AU0
Cl in 6ARJ
Cl in 6AU6
Cl in 6AU1
Cl in 6ASV
Cl in 6ATE
Cl in 6ASQ
Cl in 6ASP
Cl in 6ART