Magnesium »
PDB 5zkj-6a1l »
5zwk »
Magnesium in PDB 5zwk: Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp
Protein crystallography data
The structure of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp, PDB code: 5zwk
was solved by
H.Yunyuan,
G.Zeyuan,
Y.Junjie,
Y.Ping,
W.Jian,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.13 / 2.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 66.006, 155.746, 76.082, 90.00, 115.54, 90.00 |
| R / Rfree (%) | 19.4 / 24.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp
(pdb code 5zwk). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 8 binding sites of Magnesium where determined in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp, PDB code: 5zwk:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Magnesium where determined in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp, PDB code: 5zwk:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Magnesium binding site 1 out of 8 in 5zwk
Go back to
Magnesium binding site 1 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp
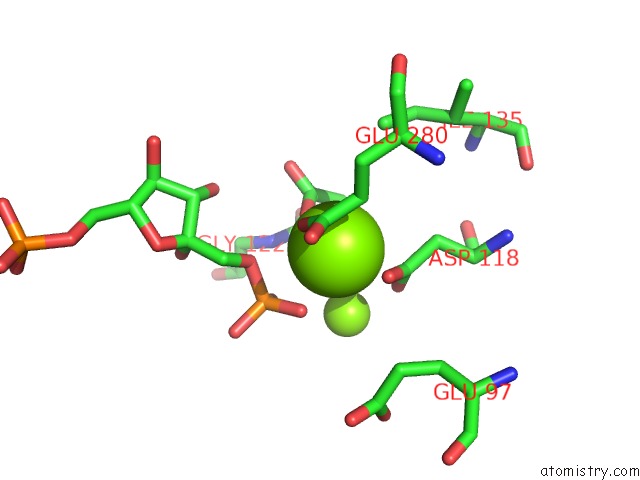
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 2 out of 8 in 5zwk
Go back to
Magnesium binding site 2 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 3 out of 8 in 5zwk
Go back to
Magnesium binding site 3 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 4 out of 8 in 5zwk
Go back to
Magnesium binding site 4 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 5 out of 8 in 5zwk
Go back to
Magnesium binding site 5 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 6 out of 8 in 5zwk
Go back to
Magnesium binding site 6 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp
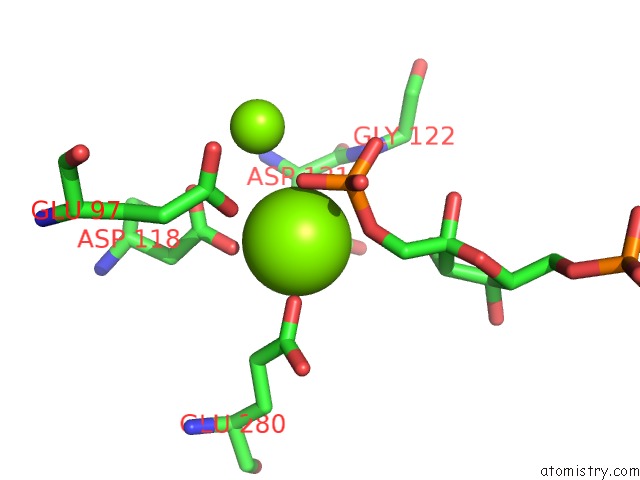
Mono view
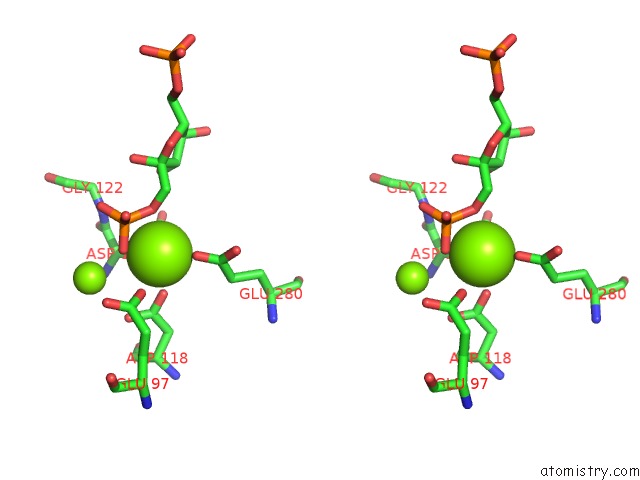
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 7 out of 8 in 5zwk
Go back to
Magnesium binding site 7 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp
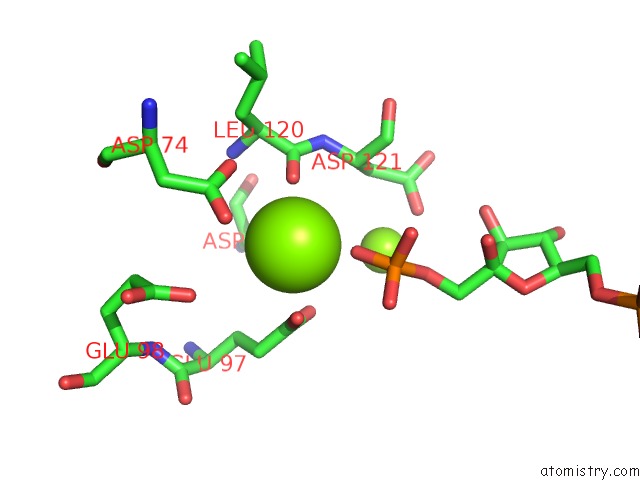
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Magnesium binding site 8 out of 8 in 5zwk
Go back to
Magnesium binding site 8 out
of 8 in the Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Crystal Structure of Human Liver Fructose-1,6-Bisphoaphatase Complex with Fructose-1,6-Bisphophate and Amp within 5.0Å range:
|
Reference:
H.Yunyuan,
G.Zeyuan,
Y.Junjie,
Y.Ping,
W.Jian,
R.Li.
Location of Fbpase Catalytic Metal Binding Site: A Combined Experimental and Theoretical Study To Be Published.
Page generated: Mon Sep 30 18:44:26 2024
Last articles
Ca in 5PANCa in 5PAM
Ca in 5PAK
Ca in 5PAJ
Ca in 5PAI
Ca in 5PAG
Ca in 5PAF
Ca in 5P2P
Ca in 5PAC
Ca in 5PAE